
CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES ›› 2024, Vol. 42 ›› Issue (2): 169-176.doi: 10.12140/j.issn.1000-7423.2024.02.006
• ORIGINAL ARTICLES • Previous Articles Next Articles
TAN Nie1( ), JIAO Shiming1,2, DING Yan1, ZHU Chengyu2, XU Wenyue1,2,*(
), JIAO Shiming1,2, DING Yan1, ZHU Chengyu2, XU Wenyue1,2,*( )
)
Received:2023-11-01
Revised:2023-12-26
Online:2024-04-30
Published:2024-04-26
Contact:
* E-mail: Supported by:CLC Number:
TAN Nie, JIAO Shiming, DING Yan, ZHU Chengyu, XU Wenyue. Effect of local complement activation in hepatocytes on the development of Plasmodium in the infrared phase[J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2024, 42(2): 169-176.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.jsczz.cn/EN/10.12140/j.issn.1000-7423.2024.02.006
Table 1
PCR amplification primer sequences for complement C3, C5 and their receptor genes
| 来源 Source | 基因Gene | 引物序列(5'→3') Primer sequence (5'→3') |
|---|---|---|
| 小鼠 Mus musculus | C3 | F:CACACCGAAGAAGACTGCCTGAC R:CTGACTTGATGACCTGCTGGATGG |
| C3aR1 | F:TCTCCTTGGCTCACCTGATTCTCC R:AGGCTACCACCCAGACACATCC | |
| C5 | F:TACAAGCCCAGCAAGGAGGAGTC R:AGGCTACCACCCAGACACATCC | |
| C5aR1 | F:TCTACTCGGTGGTGTTCCTGGTG R:AAGGATGGAATGGTGAGGAGCAATG | |
| 人 Homo sapiens | C3 | F:GTCACTGTTACTGTCCACGACTTCC R:GCACCACCTTCTCCACCACTTG |
| C3aR1 | F:GCAGCGGACAGTGAACACAATTTG R:CACAAGCCACCACCCAGATACATC | |
| C5 | F:TTCAACCCAGGACACCATCAATGC R:TGTAGCCAAGCCACTGCCAAATC | |
| C5aR1 | F:AAGCGGACCATCAATGCCATCTG R:TCCACGCCACACAACACCTTTG |
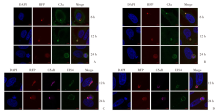
Fig. 2
Laser confocal microscope observation of hepatocyte local complement enrichment around parasitophorous vacuole (Indirect immunofluorescence staining, × 2 500) A: The expression of C3a around the parasitophorous vacuole. B: The expression of C5a around the parasitophorous vacuole. C: The expression of C5aR on the parasitophorous vacuole membrane. D: The expression of C5aR on the parasitophorous vacuole membrane after incubating with C5aR antagonist. The nucleus shows blue fluorescence by DAPI; P. yoelii exprsses RFP in infrared phase, showing red spontaneous fluorescence; C3a, C5a shows green fluorescence inside hepatocytes; C5aR enriches on the vacuolar membrane of nanoworms, exhibiting pink fluorescence; UIS4 shows green fluorescence on the vacuolar membrane of the nanoworm; Merge means merge images.
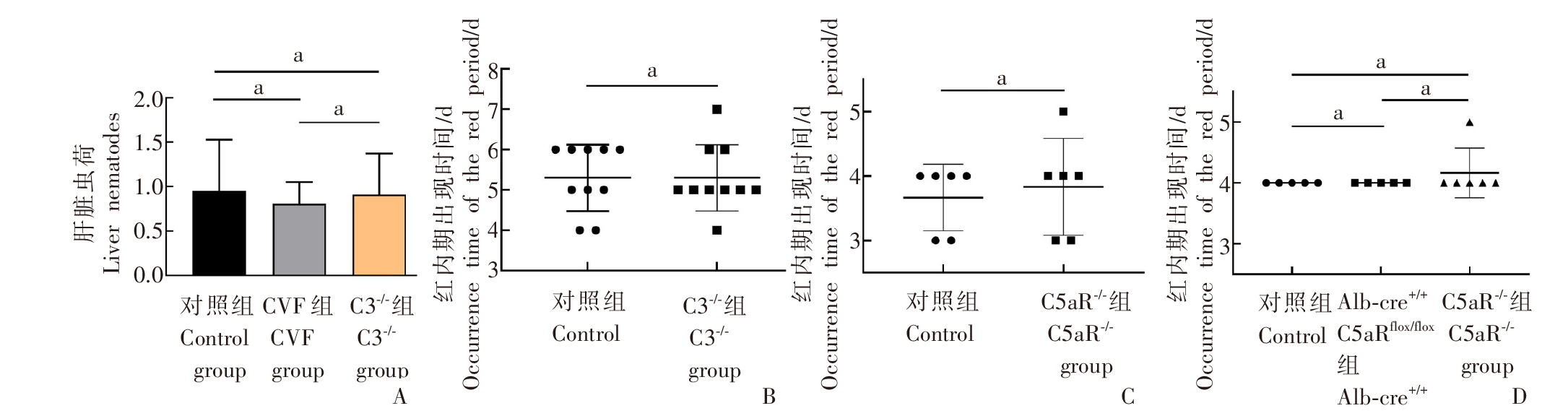
Fig. 3
The effects of knocking out different complements on the liver parasites infrared and phase development of Plasmodium A: Liver parasites of mice infected with P. yoelii in the control group, CVF group and C3-/- group; B: Appearance time of the red phase after attacking the control group and C3aR-/- mice with P. yoelii; C: Appearance time of the red phase after attacking the control group and C5aR-/- mice with P. berghei; D: The appearance time of the red phase after P. yoelii attacked Alb-cre+/+C5aRflox/flox hybrid mice and C5aR-/- mice. a: P > 0.05.
| [1] | WHO. World malaria report 2023[R]. Geneva: World Health Organization, 2023: 21, 78, 80. |
| [2] | Noedl H, Se Y, Schaecher K, et al. Evidence of artemisinin-resistant malaria in western Cambodia[J]. N Engl J Med, 2008, 359(24): 2619-2620. |
| [3] | Balikagala B, Fukuda N, Ikeda M, et al. Evidence of artemisinin-resistant malaria in Africa[J]. N Engl J Med, 2021, 385(13): 1163-1171. |
| [4] |
Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease[J]. Annu Rev Entomol, 2000, 45: 371-391.
pmid: 10761582 |
| [5] | Zhu GD, Cao J. Regular assessment is an effective approach to maintaining the capacity of prevention of re-establishment from imported malaria in China[J]. Chin J Schisto Control, 2023, 9(2): 113-115, 120. (in Chinese) |
| (朱国鼎, 曹俊. 朝督暮责: 定期开展评估是保持防止疟疾输入再传播能力的有效手段[J]. 中国血吸虫病防治杂志, 2023, 9(2): 113-115, 120.) | |
| [6] | Zhu GD, Gao Q, Cao J. Challenges and countermeasures in prevention of re-establishment of imported malaria in China[J]. Chin J Schisto Control, 2021, 33(1): 7-9, 21. (in Chinese) |
| (朱国鼎, 高琪, 曹俊. 中国防止疟疾输入再传播面临的挑战和应对策略[J]. 中国血吸虫病防治杂志, 2021, 33(1): 7-9, 21.) | |
| [7] | Xu WY. Development and application of the world’s first malaria subunit vaccine RTS, S/AS01[J]. Chin J Schisto Control, 2021, 7(6): 557-559. (in Chinese) |
| (徐文岳. 全球首款疟疾疫苗RTS, S/AS01的研发和应用[J]. 中国血吸虫病防治杂志, 2021, 7(6): 557-559.) | |
| [8] | Stewart MJ, Vanderberg JP. Malaria sporozoites leave behind trails of circumsporozoite protein during gliding motility[J]. J Protozool, 1988, 35(3): 389-393. |
| [9] |
Mota MM, Pradel G, Vanderberg JP, et al. Migration of Plasmodium sporozoites through cells before infection[J]. Science, 2001, 291(5501): 141-144.
doi: 10.1126/science.291.5501.141 pmid: 11141568 |
| [10] |
Phillips MA, Burrows JN, Manyando C, et al. Malaria[J]. Nat Rev Dis Primers, 2017, 3: 17050.
doi: 10.1038/nrdp.2017.50 pmid: 28770814 |
| [11] |
Amino R, Giovannini D, Thiberge S, et al. Host cell traversal is important for progression of the malaria parasite through the dermis to the liver[J]. Cell Host Microbe, 2008, 3(2): 88-96.
doi: 10.1016/j.chom.2007.12.007 pmid: 18312843 |
| [12] |
Amino R, Thiberge S, Blazquez S, et al. Imaging malaria sporozoites in the dermis of the mammalian host[J]. Nat Protoc, 2007, 2(7): 1705-1712.
pmid: 17641635 |
| [13] | Amino R, Thiberge S, Martin B, et al. Quantitative imaging of Plasmodium transmission from mosquito to mammal[J]. Nat Med, 2006, 12(2): 220-224. |
| [14] |
Gueirard P, Tavares J, Thiberge S, et al. Development of the malaria parasite in the skin of the mammalian host[J]. Proc Natl Acad Sci USA, 2010, 107(43): 18640-18645.
doi: 10.1073/pnas.1009346107 pmid: 20921402 |
| [15] |
Voza T, Miller JL, Kappe SH, et al. Extrahepatic exoerythrocytic forms of rodent malaria parasites at the site of inoculation: clearance after immunization, susceptibility to primaquine, and contribution to blood-stage infection[J]. Infect Immun, 2012, 80(6): 2158-2164.
doi: 10.1128/IAI.00246-12 pmid: 22431651 |
| [16] |
Risco-Castillo V, Topçu S, Marinach C, et al. Malaria sporozoites traverse host cells within transient vacuoles[J]. Cell Host Microbe, 2015, 18(5): 593-603.
pmid: 26607162 |
| [17] |
Pradel G, Frevert U. Malaria sporozoites actively enter and pass through rat Kupffer cells prior to hepatocyte invasion[J]. Hepatology, 2001, 33(5): 1154-1165.
pmid: 11343244 |
| [18] | Mota MM, Hafalla JC, Rodriguez A. Migration through host cells activates Plasmodium sporozoites for infection[J]. Nat Med, 2002, 8(11): 1318-1322. |
| [19] |
Carrolo M, Giordano S, Cabrita-Santos L, et al. Hepatocyte growth factor and its receptor are required for malaria infection[J]. Nat Med, 2003, 9(11): 1363-1369.
pmid: 14556002 |
| [20] | Frevert U, Engelmann S, Zougbédé S, et al. Intravital observation of Plasmodium berghei sporozoite infection of the liver[J]. PLoS Biol, 2005, 3(6): e192. |
| [21] |
Pradel G, Garapaty S, Frevert U. Proteoglycans mediate malaria sporozoite targeting to the liver[J]. Mol Microbiol, 2002, 45(3): 637-651.
pmid: 12139612 |
| [22] |
Sturm A, Graewe S, Franke-Fayard B, et al. Alteration of the parasite plasma membrane and the parasitophorous vacuole membrane during exo-erythrocytic development of malaria parasites[J]. Protist, 2009, 160(1): 51-63.
doi: 10.1016/j.protis.2008.08.002 pmid: 19026596 |
| [23] |
Rodrigues CD, Hannus M, Prudêncio M, et al. Host scavenger receptor SR-BI plays a dual role in the establishment of malaria parasite liver infection[J]. Cell Host Microbe, 2008, 4(3): 271-282.
doi: 10.1016/j.chom.2008.07.012 pmid: 18779053 |
| [24] | Yalaoui S, Zougbédé S, Charrin S, et al. Hepatocyte permissiveness to Plasmodium infection is conveyed by a short and structurally conserved region of the CD81 large extracellular domain[J]. PLoS Pathog, 2008, 4(2): e1000010. |
| [25] | Silvie O, Greco C, Franetich JF, et al. Expression of human CD81 differently affects host cell susceptibility to malaria sporozoites depending on the Plasmodium species[J]. Cell Microbiol, 2006, 8(7): 1134-1146. |
| [26] |
Kiyuka PK, Meri S, Khattab A. Complement in malaria: Immune evasion strategies and role in protective immunity[J]. FEBS Lett, 2020, 594(16): 2502-2517.
doi: 10.1002/1873-3468.13772 pmid: 32181490 |
| [27] |
Simon N, Lasonder E, Scheuermayer M, et al. Malaria parasites co-opt human factor H to prevent complement-mediated lysis in the mosquito midgut[J]. Cell Host Microbe, 2013, 13(1): 29-41.
doi: 10.1016/j.chom.2012.11.013 pmid: 23332154 |
| [28] | Kennedy AT, Schmidt CQ, Thompson JK, et al. Recruitment of factor H as a novel complement evasion strategy for blood-stage Plasmodium falciparum infection[J]. J Immunol, 2016, 196(3): 1239-1248. |
| [29] | Kennedy AT, Wijeyewickrema LC, Huglo A, et al. Recruitment of human C1 esterase inhibitor controls complement activation on blood stage Plasmodium falciparum merozoites[J]. J Immunol, 2017, 198(12): 4728-4737. |
| [30] |
Kurtovic L, Behet MC, Feng GQ, et al. Human antibodies activate complement against Plasmodium falciparum sporozoites, and are associated with protection against malaria in children[J]. BMC Med, 2018, 16(1): 61.
doi: 10.1186/s12916-018-1054-2 pmid: 29706136 |
| [31] | Boyle MJ, Reiling L, Feng GQ, et al. Human antibodies fix complement to inhibit Plasmodium falciparum invasion of erythrocytes and are associated with protection against malaria[J]. Immunity, 2015, 42(3): 580-590. |
| [32] |
Reiling L, Boyle MJ, White MT, et al. Targets of complement-fixing antibodies in protective immunity against malaria in children[J]. Nat Commun, 2019, 10(1): 610.
doi: 10.1038/s41467-019-08528-z pmid: 30723225 |
| [33] | Behet MC, Kurtovic L, van Gemert GJ, et al. The complement system contributes to functional antibody-mediated responses induced by immunization with Plasmodium falciparum malaria sporozoites[J]. Infect Immun, 2018, 86(7): e00920-e00917. |
| [34] | Arbore G, Kemper C, Kolev M. Intracellular complement—the complosome—in immune cell regulation[J]. Mol Immunol, 2017, 89: 2-9. |
| [35] | Kolev M le Friec G, Kemper C. Complement: tapping into new sites and effector systems[J]. Nat Rev Immunol, 2014, 14(12): 811-820. |
| [36] |
Liszewski MK, Kolev M le Friec G, et al. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation[J]. Immunity, 2013, 39(6): 1143-1157.
doi: 10.1016/j.immuni.2013.10.018 pmid: 24315997 |
| [37] | Tam JC, Bidgood SR, McEwan WA, et al. Intracellular sensing of complement C3 activates cell autonomous immunity[J]. Science, 2014, 345(6201): 1256070. |
| [38] |
Khan ZM, Vanderberg JP. Role of host cellular response in differential susceptibility of nonimmunized BALB/c mice to Plasmodium berghei and Plasmodium yoelii sporozoites[J]. Infect Immun, 1991, 59(8): 2529-2534.
doi: 10.1128/iai.59.8.2529-2534.1991 pmid: 1855974 |
| [39] | Silvie O, Rubinstein E, Franetich JF, et al. Hepatocyte CD81 is required for Plasmodium falciparum and Plasmodium yoelii sporozoite infectivity[J]. Nat Med, 2003, 9(1): 93-96. |
| [40] |
Lalli PN, Strainic MG, Yang M, et al. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis[J]. Blood, 2008, 112(5): 1759-1766.
doi: 10.1182/blood-2008-04-151068 pmid: 18567839 |
| [41] |
Zenklusen I, Jongo S, Abdulla S, et al. Immunization of malaria-preexposed volunteers with PfSPZ vaccine elicits long-lived IgM invasion-inhibitory and complement-fixing antibodies[J]. J Infect Dis, 2018, 217(10): 1569-1578.
doi: 10.1093/infdis/jiy080 pmid: 29438525 |
| [42] |
Kawamoto Y, Winger LA, Hong K, et al. Plasmodium berghei: Sporozoites are sensitive to human serum but not susceptible host serum[J]. Exp Parasitol, 1992, 75(3): 361-368.
pmid: 1426138 |
| [43] |
Huber-Lang M, Sarma JV, Zetoune FS, et al. Generation of C5a in the absence of C3: a new complement activation pathway[J]. Nat Med, 2006, 12(6): 682-687.
doi: 10.1038/nm1419 pmid: 16715088 |
| [44] |
Ramos TN, Darley MM, Weckbach S, et al. The C5 convertase is not required for activation of the terminal complement pathway in murine experimental cerebral malaria[J]. J Biol Chem, 2012, 287(29): 24734-24738.
doi: 10.1074/jbc.C112.378364 pmid: 22689574 |
| [45] | Ernest M, Rosa TFA, Pala ZR, et al. Plasmodium falciparum gametes and sporozoites hijack plasmin and factor H to evade host complement killing[J]. Microbiol Spectr, 2023, 11(3): e0449322. |
| [46] | Schmidt CQ, Kennedy AT, Tham WH. More than just immune evasion: hijacking complement by Plasmodium falciparum[J]. Mol Immunol, 2015, 67(1): 71-84. |
| [47] | Atkinson JP, Glew RH, Neva FA, et al. Serum complement and immunity in experimental simian malaria. Ⅱ. Preferential activation of early components and failure of depletion of late components to inhibit protective immunity[J]. J Infect Dis, 131(1): 26-33. |
| [48] |
Ramos TN, Bullard DC, Barnum SR. Deletion of the complement phagocytic receptors CR3 and CR4 does not alter susceptibility to experimental cerebral malaria[J]. Parasite Immunol, 2012, 34(11): 547-550.
doi: 10.1111/pim.12002 pmid: 22882618 |
| [1] | HAN Zhuxi, ZHU Xiaotong. Research progress on Plasmodium membrane protein complexes [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2024, 42(1): 111-116. |
| [2] | GUO Shuai, HE Biao, GAO Yuanli, FAN Yongling, ZHU Feng, DING Yan, LIU Taiping, XU Wenyue. Specie-specific analysis of plasmodia infecting rats and mice [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(5): 539-545. |
| [3] | LIANG Kejia, LIU Cong, LI Yanlin, LI Xiaoge, LIU Yan, LI Zhenkui. Research advances on transcriptional regulation in plasmodium sexual stages [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(5): 619-624. |
| [4] | SUN Jun. The biological significance of malarial hemozoin’s formation [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(2): 209-212. |
| [5] | GE Jie-yun, LIU Lei, SUN Yi-fan, CHENG Yang. Advances in research on the vacuolar membrane function and the associated proteins of plasmodium parasitophorous vacuole [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(3): 402-410. |
| [6] | LU Fei, ZHUO Xun-hui, LU Shao-hong. Research progress on the interaction between host cell autophagy and apicomplexa protozoa infection [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(6): 826-831. |
| [7] | LI Mei, TU Hong, XIA Zhi-gui, WANG Zhen-yu, ZHOU He-jun. Thermal stability of diagnostic targets Plasmodium falciparum histidine rich protein Ⅱ and Plasmodium lactate dehydrogenase in rapid detection [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(2): 245-248. |
| [8] | LIU Hong, LIU Yao-bao, CAO Jun. Research advance and application of whole-genome sequencing of Plasmodium [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(2): 265-270. |
| [9] | LUO Fei, TAN Yan, ZHOU Shuang, YUAN Yi, LI Shan-shan, XU Jing-ru, ZHOU Yang. Assessment and analysis of malaria diagnostic capacity by microscopy at primary health institutions in Chongqing [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(1): 93-99. |
| [10] | ZHU Ling-qian, FENG Xin-yu, HU Wei, LI Shi-zhu. Functions and roles of miRNA during the infection of Anopheles by Plasmodium [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2020, 38(6): 742-748. |
| [11] | ZHOU Shui-mao, TU Zu-wu, YANG Yan, CHEN Fang, JIA Xi-shuai. Evaluation of the efficacy of loop-mediated isothermal amplification in detecting Plasmodium falciparum and other species of Plasmodium [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2020, 38(4): 423-428. |
| [12] | ZHANG Yan, XU Ai-fang, ZHANG Jia-qi, YAO Li-nong, GU Kai-long, XUE Li-zhi, PAN Ke-nv. Differential diagnosis of a case of Babesia microti infection previously misdiagnosed as malaria [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2020, 38(4): 445-448. |
| [13] | Wen-qi ZHENG, Xue-min FENG, Ya-ming CAO, Yan-qiu HAN, Jun-rui WANG. Bioinformatics analysis and enzymatic activity test of quiescin sulfhydryl oxidase from Plasmodium berghei [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2020, 38(2): 159-165. |
| [14] | Rui YANG, Yu-ting ZHENG, Xiao-yu YANG, Li-min DONG, Zu-rui LIN, Yao-wu ZHOU, Xu-can ZENG, Hong-bin LI, Jin-yong JIANG. Investigation on malaria vectors in Jinghong, a border area in Yunnan Province [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2019, 37(4): 406-410. |
| [15] | Ji-hong CAO, Zi-long ZHANG, Shen-wei LI, Mei LI, Xiao-hang ZHANG, Zhen-gan TIAN. Application of high-throughput DNA microarray for rapid detection of Plasmodium spp. at port [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2019, 37(1): 61-65. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||