
CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES ›› 2022, Vol. 40 ›› Issue (6): 708-716.doi: 10.12140/j.issn.1000-7423.2022.06.003
• ORIGINAL ARTICLES • Previous Articles Next Articles
HOU Xin-ling1,2( ), LI De-wei1,2, SHI Yang1,2, WANG Mao-lin1,2, ZIBIGU Rousu1,2, ABIDAN Ainiwaer1,2, ZHENG Xu-ran1,2, KANG Xue-jiao1,2, WANG Hui1,2, LI Jing1,2, ZHANG Chuan-shan1,2(
), LI De-wei1,2, SHI Yang1,2, WANG Mao-lin1,2, ZIBIGU Rousu1,2, ABIDAN Ainiwaer1,2, ZHENG Xu-ran1,2, KANG Xue-jiao1,2, WANG Hui1,2, LI Jing1,2, ZHANG Chuan-shan1,2( )
)
Received:2022-03-28
Revised:2022-09-16
Online:2022-12-30
Published:2022-12-26
Contact:
ZHANG Chuan-shan
E-mail:465778824@qq.com;dashan0518@126.com
Supported by:CLC Number:
HOU Xin-ling, LI De-wei, SHI Yang, WANG Mao-lin, ZIBIGU Rousu, ABIDAN Ainiwaer, ZHENG Xu-ran, KANG Xue-jiao, WANG Hui, LI Jing, ZHANG Chuan-shan. Changes of ST2+ T cell subset function and their immune checkpoint molecule expression in the peritoneal cavity of mice infected with Echinococcus multilocularis[J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(6): 708-716.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.jsczz.cn/EN/10.12140/j.issn.1000-7423.2022.06.003
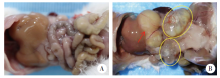
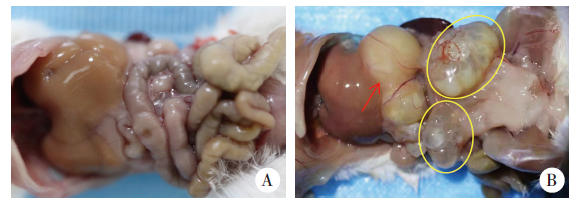
Fig. 1
Peritoneal lesions in mice infected with E. multilocularis A:Control group, abdominal viscera presents normal morphology;B:Infection group, cystic or solid lesions can be seen in the abdominal cavity. The red arrow indicates the liver lesions, and the yellow circles indicate the abdominal metastatic lesion.
| [1] | Wu WP, Wang H, Wang Q, et al. A nationwide sampling survey on echinococcosis in China during 2012—2016[J]. Chin J Parasitol Parasit Dis, 2018, 36(1): 1-14. (in Chinese) |
| (伍卫平, 王虎, 王谦, 等. 2012—2016年中国棘球蚴病抽样调查分析[J]. 中国寄生虫学与寄生虫病杂志, 2018, 36(1): 1-14.) | |
| [2] | Wen H, Vuitton L, Tuxun T, et al. Echinococcosis: advances in the 21st century[J]. Clin Microbiol Rev, 2019, 32(2): e00075-e00018. |
| [3] | Wen H, Tuerganaili A, Shao YM, et al. Research achievements and challenges for echinococcosis control[J]. Chin J Parasitol Parasit Dis, 2015, 33(6): 466-471. (in Chinese) |
| (温浩, 吐尔干艾力·阿吉, 邵英梅, 等. 棘球蚴病防治成就及面临的挑战[J]. 中国寄生虫学与寄生虫病杂志, 2015, 33(6): 466-471.) | |
| [4] |
Gottstein B, Wang JH, Boubaker G, et al. Susceptibility versus resistance in alveolar echinococcosis (larval infection with Echinococcus multilocularis)[J]. Vet Parasitol, 2015, 213(3/4): 103-109.
doi: 10.1016/j.vetpar.2015.07.029 |
| [5] | Lachenmayer A, Gebbers D, Gottstein B, et al. Elevated incidence of alveolar echinococcosis in immunocompromised patients[J]. Food Waterborne Parasitol, 2019, 16: e00060. |
| [6] |
Zhang C, Lin R, Li Z, et al. Immune exhaustion of T cells in alveolar echinococcosis patients and its reversal by blocking checkpoint receptor TIGIT in a murine model[J]. Hepatology, 2020, 71(4): 1297-1315.
doi: 10.1002/hep.30896 pmid: 31410870 |
| [7] |
Paul WE, Zhu JF. How are TH2-type immune responses initiated and amplified?[J]. Nat Rev Immunol, 2010, 10(4): 225-235.
doi: 10.1038/nri2735 |
| [8] |
Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines[J]. Immunity, 2005, 23(5): 479-490.
doi: 10.1016/j.immuni.2005.09.015 pmid: 16286016 |
| [9] | Rostan O, Gangneux JP, Piquet-Pellorce C, et al. The IL-33/ST2 axis is associated with human visceral leishmaniasis and suppresses Th1 responses in the livers of BALB/c mice infected with Leishmania donovani[J]. mBio, 2013, 4(5): e00383-e00313. |
| [10] |
Ryan N, Anderson K, Volpedo G, et al. The IL-33/ST2 axis in immune responses against parasitic disease: potential therapeutic applications[J]. Front Cell Infect Microbiol, 2020, 10: 153.
doi: 10.3389/fcimb.2020.00153 |
| [11] |
An MT, Zhu YJ, Xu C, et al. Soluble ST2 (sST2) as potential marker for hepatic cystic echinococcosis activity[J]. J Infect, 2020, 80(4): 462-468.
doi: S0163-4453(20)30058-X pmid: 32027871 |
| [12] | Kushwaha JK, Sonkar AA, Verma AK, et al. Primary disseminated extrahepatic abdominal hydatid cyst: a rare disease[J]. BMJ Case Rep, 2012, 2012: bcr0220125808. |
| [13] |
Zhang CS, Shao YM, Yang ST, et al. T-cell tolerance and exhaustion in the clearance of Echinococcus multilocularis: role of inoculum size in a quantitative hepatic experimental model[J]. Sci Rep, 2017, 7: 11153.
doi: 10.1038/s41598-017-11703-1 |
| [14] |
Frey AB. Suppression of T cell responses in the tumor microenvironment[J]. Vaccine, 2015, 33(51): 7393-7400.
doi: S0264-410X(15)01281-5 pmid: 26403368 |
| [15] |
Lodygin D, Flügel A. Intravital real-time analysis of T-cell activation in health and disease[J]. Cell Calcium, 2017, 64: 118-129.
doi: S0143-4160(16)30230-5 pmid: 28126314 |
| [16] |
Mejri N, Gottstein B. Intraperitoneal Echinococcus multilocularis infection in C57BL/6 mice affects CD40 and B7 costimulator expression on peritoneal macrophages and impairs peritoneal T cell activation[J]. Parasite Immunol, 2006, 28(8): 373-385.
pmid: 16879309 |
| [17] | Hou XL, Li L, Li LH, et al. Exhaustion of CD8+ T cell immune functions in spleen of mice with different doses of Echinococcus multilocularis infections[J]. Chin J Schisto Control, 2020, 32(6): 591-597, 604. (in Chinese) |
| (侯昕伶, 李亮, 李玲慧, 等. 泡球蚴感染对小鼠脾脏CD8+ T细胞免疫功能耗竭的影响[J]. 中国血吸虫病防治杂志, 2020, 32(6): 591-597, 604.) | |
| [18] |
Vuitton DA, Bresson-Hadni S, Laroche L, et al. Cellular immune response in Echinococcus multilocularis infection in humans. Ⅱ. natural killer cell activity and cell subpopulations in the blood and in the periparasitic granuloma of patients with alveolar echinococcosis[J]. Clin Exp Immunol, 1989, 78(1): 67-74.
pmid: 2805425 |
| [19] |
Wang J, Gottstein B. Immunoregulation in larval Echinococcus multilocularis infection[J]. Parasite Immunol, 2016, 38(3): 182-192.
doi: 10.1111/pim.12292 pmid: 26536823 |
| [20] | Hou XL, Li LH, Li L, et al. Changes in subsets and functional exhaustion of CD4+ T cells in spleens of mice infected with Echinococcus multilocularis[J]. Chin J Parasitol Parasit Dis, 2020, 38(5): 611-618, 624. (in Chinese) |
| (侯昕伶, 李玲慧, 李亮, 等. 多房棘球蚴感染小鼠脾CD4+ T细胞亚群及其功能耗竭的变化[J]. 中国寄生虫学与寄生虫病杂志, 2020, 38(5): 611-618, 624.) | |
| [21] |
Khalid KE, Nascimento MSL, Sacramento LA, et al. T1/ST2 deficient mice display protection against Leishmania infantum experimental infection[J]. Acta Trop, 2017, 172: 1-6.
doi: 10.1016/j.actatropica.2017.04.011 |
| [22] |
Long X, Daya M, Zhao JP, et al. The role of ST2 and ST2 genetic variants in schistosomiasis[J]. J Allergy Clin Immunol, 2017, 140(5): 1416-1422.
doi: S0091-6749(17)30215-4 pmid: 28189770 |
| [23] |
Zhang YX, He J, Zheng HQ, et al. Association of TREM-1, IL-1β, IL-33/ST2, and TLR expressions with the pathogenesis of ocular toxoplasmosis in mouse models on different genetic backgrounds[J]. Front Microbiol, 2019, 10: 2264.
doi: 10.3389/fmicb.2019.02264 pmid: 31649630 |
| [24] |
Bai Y, Guan F, Zhu F, et al. IL-33/ST2 axis deficiency exacerbates hepatic pathology by regulating treg and Th17 cells in murine schistosomiasis Japonica[J]. J Inflamm Res, 2021, 14: 5981-5998.
doi: 10.2147/JIR.S336404 pmid: 34815688 |
| [25] |
Seki T, Obata-Ninomiya K, Shimogawara-Furushima R, et al. IL-33/ST2 contributes to severe symptoms in Plasmodium chabaudi-infected BALB/c mice[J]. Parasitol Int, 2018, 67(1): 64-69.
doi: 10.1016/j.parint.2017.03.008 |
| [26] |
Butler NS, Moebius J, Pewe LL, et al. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection[J]. Nat Immunol, 2012, 13(2): 188-195.
doi: 10.1038/ni.2180 |
| [27] |
Li L, Wan S, Tao K, et al. KLRG1 restricts memory T cell antitumor immunity[J]. Oncotarget, 2016, 7(38): 61670-61678.
doi: 10.18632/oncotarget.11430 pmid: 27557510 |
| [28] |
Nono JK, Lutz MB, Brehm K. Expansion of host regulatory T cells by secreted products of the tapeworm Echinococcus multilocularis[J]. Front Immunol, 2020, 11: 798.
doi: 10.3389/fimmu.2020.00798 |
| [29] | Wang J, Jebbawi F, Bellanger AP, et al. Immunotherapy of alveolar echinococcosis via PD-1/PD-L1 immune checkpoint blockade in mice[J]. Parasite Immunol, 2018, 40(12): e12596. |
| [30] | Bellanger AP, Courquet S, Pallandre JR, et al. Echinococcus multilocularis vesicular fluid induces the expression of immune checkpoint proteins in vitro[J]. Parasite Immunol, 2020, 42(6): e12711. |
| [1] | CAO Deping, LI Jiajing, SONG Mengwei, MO Gang. Experimental observation on the changes of hepatic stellate cells stimulated in vitro with tissue protein of Echinococcus multilocularis [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(4): 440-445. |
| [2] | DU Tao, HU Chunhui, GAN Xuehui, GAO Pan, ZHANG Fabin. Anti-Echinococcus multilocularis effect of total alkaloids of Sophora moorcroftiana in water solution and tablet forms in vitro and in vivo [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(1): 15-22. |
| [3] | WU Liang-liang, YANG Ling-fei, SONG Tao. Ultrasound and pathological manifestations of lesions in SD rats with hepatic Echinococcus multilocularis infection established by different methods [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(4): 549-552. |
| [4] | ZHONG Shun-hu, SUN Yue, GUO Xiao-la, ZHENG Ya-dong, CHEN Yi-xia. Identification and bioinformatics analysis of differentially expressed miRNAs in splenic lymphocytes in Echinococcus multilocularis-infected mice [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(3): 288-294. |
| [5] | ZHUO Yi-cheng, YANG Hai-cheng, LIU Cheng-hao, ZHANG Bao-cai, DUO Xiao-yong, ZHANG Shi-jie. Effect of osteopontin expression level on the growth and development of Echinococcus multilocularis protoscoleces [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(3): 299-304. |
| [6] | ABUDUAINI Abulizi, PAIZULA Shalayiadang, TALAITI Tuergan, ZHANG Rui-qing, WANG Hui, ZHANG Chuan-shan, SHAO Ying-mei, TUERGANAILI Aji. Affect of Echinococcus multilocularis protein-mediated NK cell surface receptor NKG2A on the function of NK cells [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(1): 36-42. |
| [7] | HOU Jiao, WEN Hao, WANG Ming-kun, LI Wen-ding, LI liang, LI Jing, ZHANG Chuan-shan, WANG Hui. Changes of macrophage subsets and polarization in spleen of mice infected with Echinococcus multilocularis [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(6): 771-778. |
| [8] | SHI Qi-qi, LIU Cong-shan, HUO Le-le, WEI Yu-fen, JIANG Bin, YIN Meng, XUE Jian, TAO Yi, ZHANG Hao-bing. Affect of aminoalcohol compound HT24 on the expression of tubulin in Echinococcus multilocularis protoscoleces [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(4): 437-443. |
| [9] | LI Ling-hui, WANG Wei, HOU Xin-ling, SHI Yang, LI De-wei, LI Liang, WANG Hui, LI Jing, ZHANG Chuan-shan. Affects of Echinococcus multilocularis metacestode infection on the natural killer T cells and their subsets in mouse spleen [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(3): 311-317. |
| [10] | GUO Bao-ping, GUO Gang, ZHANG Li, XIANG Jing-jing, WANG Xiao-ping, REN Yuan, QI Wen-jing, ZHANG Hui, LI Jun, ZHANG Wen-bao, WANG Hai-yan. Investigation on infection of Echinococcus multilocularis metacestode in small rodents in Chabchar County, Xinjiang [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(3): 327-332. |
| [11] | XU Kai, WANG Hai-jiu, ZHANG Li, ZHANG Yao-gang, FAN Hai-ning, REN Li, REN Bin, WANG Zhi-xin. Research progress on the mechanisms underlying the impairment of host hepatocytes by Echinococcus multilocularis [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(2): 256-259. |
| [12] | ZHU Ji-hai, CAO De-ping, ZHAO Jun, LIU Jun, SHI Hu-xiang, LIU Yan. Investigation of differentially expressed genes in liver tissues of patients with alveolar echinococcosis [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(1): 48-54. |
| [13] | ZHU Ji-hai, CAO De-ping, QIE Yangrangzhong, LIU Jun, ZHAO Jun, LIU Yan. Effect of Elsholtzia eriostachya in combination with albendazole in treatment of secondary Echinococcus multilocularis metacestode infection in rats [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2020, 38(6): 688-694. |
| [14] | HOU Xin-ling, LI Ling-hui, LI Liang, LI Jing, WANG Hui, SHAO Ying-mei, ZHANG Chuan-shan. Changes in subsets and functional exhaustion of CD4+ T cells in spleens of mice infected with Echinococcus multilocularis [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2020, 38(5): 611-618. |
| [15] | SHANG Jing-ye, ZHANG Guang-jia, YU Wen-jie, HE Wei, LIAO Sha, LI Rui-rui, HUANG Yan, LIU Yang, ZHONG Bo. Advances in researches on the genetic diversity of Echinococcus multilocularis [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2020, 38(5): 637-641. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||