
CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES ›› 2022, Vol. 40 ›› Issue (6): 701-707.doi: 10.12140/j.issn.1000-7423.2022.06.002
• ORIGINAL ARTICLES • Previous Articles Next Articles
WANG Xiao-ling1( ), ZHANG Wei2, YI Cun2, CHEN Xiang-yu2, YANG Wen-bin2, XU Bin1, HU Wei1,2,3(
), ZHANG Wei2, YI Cun2, CHEN Xiang-yu2, YANG Wen-bin2, XU Bin1, HU Wei1,2,3( )
)
Received:2022-06-23
Revised:2022-07-15
Online:2022-12-30
Published:2022-11-24
Contact:
HU Wei
E-mail:xwang7@126.com;huw@fudan.edu.cn
Supported by:CLC Number:
WANG Xiao-ling, ZHANG Wei, YI Cun, CHEN Xiang-yu, YANG Wen-bin, XU Bin, HU Wei. The effect of SjGPR89 protein on the growth and development of Schistosoma japonicum[J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(6): 701-707.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.jsczz.cn/EN/10.12140/j.issn.1000-7423.2022.06.002
Table 1
Primer sequences for dsRNA and qRT-PCR
| 基因名称 Gene name | 引物序列(5′→3′) Primer sequence (5′→3′) |
|---|---|
| dsSjGPR89-F | TAATACGACTCACTATAGGGAGACGGCGTTAGGAGATTCAT |
| dsSjGPR89-R | TAATACGACTCACTATAGGGAGAGTCATTAGCGACTTCACATT |
| dsGFP-F | TAATACGACTCACTATAGGGAGAAGTCAGTGGAGAGGGTGAAG |
| dsGFP-R | TAATACGACTCACTATAGGGAGAACTAGTTGAACGGATCCATC |
| qSjGPR89-F | TAGACCATTGTGCTGTGAT |
| qSjGPR89-R | ACTCATACATTCGTCAGACT |
| qPSMD-F | CCTCACCAACAATTTCCACATCT |
| qPSMD-R | GATCACTTATAGCCTTGCGAACAT |
| qPSMD-F | AACAGGGTGGTGGACCTCAT |
| qPSMD-R | AGTTGGGATAGGGCCTCTCTT |
| qCollagenⅠ-F | CATGTTCAGCTTTGTGGACCT |
| qCollagenⅠ-R | GCAGCTGACTTCAGGGATGT |
| qCollagen Ⅲ-F | TCCCCTGGAATCTGTGAATC |
| qCollagen Ⅲ-R | TGAGTCGAATTGGGGAGTAAT |
| qα-SMA-F | CCCACCCAGAGTGGAGAA |
| qα-SMA-R | ACATAGCTGGAGCAGCGTCT |
| qGAPDH-F | AACAGGGTGGTGGACCTCAT |
| qGAPDH-R | AGTTGGGATAGGGCCTCTCTT |

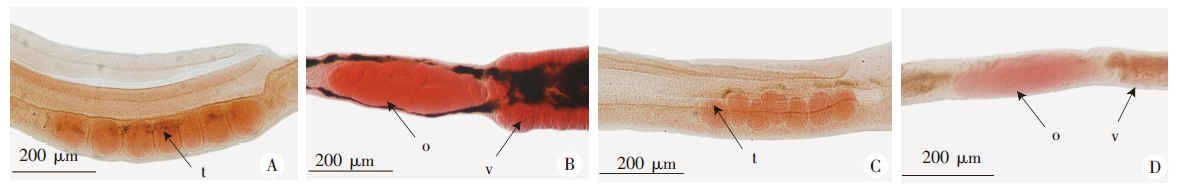
Fig. 2
Continuous interference to SjGPR89 affects gonadal development in S. japonicum (Carmin stainning, × 4) A: Male testis in control group,morphologically normal; B: Female glands in control group,morphologically normal,darker coloring; C: Male testis in the interference group, testicular diminution; D: Female glands in the interference group, development delayed, lighter in color. t: Testis; o: Ovary; v: Vitelline gland.
| [1] |
McManus DP, Bergquist R, Cai PF, et al. Schistosomiasis-from immunopathology to vaccines[J]. Semin Immunopathol, 2020, 42(3): 355-371.
doi: 10.1007/s00281-020-00789-x pmid: 32076812 |
| [2] |
Colley DG, Bustinduy AL, Secor WE, et al. Human schistosomiasis[J]. Lancet, 2014, 383(9936): 2253-2264.
doi: 10.1016/S0140-6736(13)61949-2 pmid: 24698483 |
| [3] |
Afshin A, Sur PJ, Fay KA, et al. Health effects of dietary risks in 195 countries, 1990—2017: a systematic analysis for the Global Burden of Disease Study 2017[J]. Lancet, 2019, 393(10184): 1958-1972.
doi: 10.1016/S0140-6736(19)30041-8 |
| [4] | Zhang LJ, Xu ZM, Yang F, et al. Endemic status of schistosomiasis in People’s republic of China in 2020[J]. Chin J Schisto Control, 2021, 33(3): 225-233. (in Chinese) |
| (张利娟, 徐志敏, 杨帆, 等. 2020年全国血吸虫病疫情通报[J]. 中国血吸虫病防治杂志, 2021, 33(3): 225-233.) | |
| [5] |
Moore DV, Sandground JH. The relative egg producing capacity of Schistosoma mansoni and Schistosoma japonicum[J]. Am J Trop Med Hyg, 1956, 5(5): 831-840.
doi: 10.4269/ajtmh.1956.5.831 |
| [6] | Zhou XN, Bergquist R, Leonardo L, et al. Schistosomiasis japonica control and research needs[J]. Adv Parasitol, 2010, 72: 145-178. |
| [7] |
Rask-Andersen M, Masuram S, Schiöth HB. The druggable genome: evaluation of drug targets in clinical trials suggests major shifts in molecular class and indication[J]. Annu Rev Pharmacol Toxicol, 2014, 54: 9-26.
doi: 10.1146/annurev-pharmtox-011613-135943 pmid: 24016212 |
| [8] |
Oprea TI, Bologa CG, Brunak S, et al. Unexplored therapeutic opportunities in the human genome[J]. Nat Rev Drug Discov, 2018, 17(5): 317-332.
doi: 10.1038/nrd.2018.14 pmid: 29472638 |
| [9] |
Hutchings CJ. A review of antibody-based therapeutics targeting G protein-coupled receptors: an update[J]. Expert Opin Biol Ther, 2020, 20(8): 925-935.
doi: 10.1080/14712598.2020.1745770 pmid: 32264722 |
| [10] | Wang N, Wang J. Advances in R & D of antibody drugs targeting G protein-coupled receptors[J]. Prog Pharm Sci, 2017, 41(6): 419-426. (in Chinese) |
| (王楠, 王菊. 针对GPCR蛋白家族的抗体药物研发进展[J]. 药学进展, 2017, 41(6): 419-426.) | |
| [11] |
Eiger DS, Pham U, Gardner J, et al. GPCR systems pharmacology: a different perspective on the development of biased therapeutics[J]. Am J Physiol, 2022, 322(<W>5 Pt 1):C887-C895.
doi: 10.1152/ajpcell.00449.2021 |
| [12] |
Pandey S, Nelson DC, Assmann SM. Two novel GPCR-type G proteins are abscisic acid receptors in Arabidopsis[J]. Cell, 2009, 136(1): 136-148.
doi: 10.1016/j.cell.2008.12.026 pmid: 19135895 |
| [13] |
Heidel AJ, Lawal HM, Felder M, et al. Phylogeny-wide analysis of social amoeba genomes highlights ancient origins for complex intercellular communication[J]. Genome Res, 2011, 21(11): 1882-1891.
doi: 10.1101/gr.121137.111 pmid: 21757610 |
| [14] |
Maeda Y, Ide T, Koike M, et al. GPHR is a novel anion channel critical for acidification and functions of the Golgi apparatus[J]. Nat Cell Biol, 2008, 10(10): 1135-1145.
doi: 10.1038/ncb1773 pmid: 18794847 |
| [15] |
Li J, Xiang MY, Zhang RX, et al. RNA interference in vivo in Schistosoma japonicum: establishing and optimization of RNAi mediated suppression of gene expression by long dsRNA in the intra-mammalian life stages of worms[J]. Biochem Biophys Res Commun, 2018, 503(2): 1004-1010.
doi: 10.1016/j.bbrc.2018.06.109 |
| [16] |
Liu S, Cai PF, Hou N, et al. Genome-wide identification and characterization of a panel of house-keeping genes in Schistosoma japonicum[J]. Mol Biochem Parasitol, 2012, 182(1/2): 75-82.
doi: 10.1016/j.molbiopara.2011.12.007 |
| [17] |
Weisz OA. Organelle acidification and disease[J]. Traffic, 2003, 4(2): 57-64.
pmid: 12559032 |
| [18] |
Piwon N, Günther W, Schwake M, et al. ClC-5 Cl: channel disruption impairs endocytosis in a mouse model for Dent’s disease[J]. Nature, 2000, 408(6810): 369-373.
doi: 10.1038/35042597 |
| [19] |
Rivinoja A, Kokkonen N, Kellokumpu I, et al. Elevated Golgi pH in breast and colorectal cancer cells correlates with the expression of oncofetal carbohydrate T-antigen[J]. J Cell Physiol, 2006, 208(1): 167-174.
pmid: 16547942 |
| [20] |
Cutler SR, Rodriguez PL, Finkelstein RR, et al. Abscisic acid: emergence of a core signaling network[J]. Annu Rev Plant Biol, 2010, 61: 651-679.
doi: 10.1146/annurev-arplant-042809-112122 pmid: 20192755 |
| [21] |
Rojas F, Silvester E, Young J, et al. Oligopeptide signaling through TbGPR89 drives trypanosome quorum sensing[J]. Cell, 2019, 176(1/2): 306-317.e16.
doi: 10.1016/j.cell.2018.10.041 |
| [22] | Sou Y, Yamaguchi J, Kameda H, et al. GPHR-mediated acidification of the Golgi lumen is essential for cholesterol biosynthesis in the brain[J]. FEBS Lett, 2022: online ahead of print (https://febs.onlinelibrary.wiley.com/doi/10.1002/1873-3468.14491). |
| [23] |
Gao J, Wang XW, Zou ZH, et al. Transcriptome analysis of the differences in gene expression between testis and ovary in green mud crab (Scylla paramamosain)[J]. BMC Genomics, 2014, 15(1): 585.
doi: 10.1186/1471-2164-15-585 |
| [24] |
Charroux B, Royet J. Mutations in the Drosophila ortholog of the vertebrate Golgi pH regulator (GPHR) protein disturb endoplasmic reticulum and Golgi organization and affect systemic growth[J]. Biol Open, 2014, 3(1): 72-80.
doi: 10.1242/bio.20137187 pmid: 24357227 |
| [25] |
Deckstein J, Appeldorn JV, Tsangarides M, et al. The Dictyostelium discoideum GPHR ortholog is an endoplasmic reticulum and Golgi protein with roles during development[J]. Eukaryotic Cell, 2015, 14(1): 41.
doi: 10.1128/EC.00208-14 |
| [1] | TAN Xiao, ZHU Qi, LIU Zhongqi, LI Jia, PENG Dingjin. Immunogenicity of Schistosoma japonicum Sj26gst mRNA vaccine candidate [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(5): 546-551. |
| [2] | LIU Huaman, Bikash Giri, FANG Chuantao, ZHENG Yameng, WU Huixin, ZENG Minhao, LI Shan, CHENG Guofeng. Identification of gender associated m6A modified circRNA in Schistosoma japonicum [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(5): 552-558. |
| [3] | LAN Weiming, XU Hui, XU Yin, QIU Tingting, XIE Shuying, DENG Fenglin, HU Shaoliang, LIU Huan, GUO Jiagang, ZENG Xiaojun. Study on early warning of high risk environment of Schistosoma japonicum infection by quantitative real-time PCR [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(4): 502-505. |
| [4] | CHEN Guo, ZHU Dan-dan, DUAN Yi-nong. Research progress of immune regulation protein B7 family on immune regulation during Schistosoma japonicum infection [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(6): 774-779. |
| [5] | YAN Xiao-lan, WEN Li-yong, XIONG Yan-hong, ZHENG Bin, ZHANG Jian-feng, WANG Tian-ping, YU Li-ling, XU Guo-zhang, LIN Dan-dan, ZHOU Xiao-nong. Interpretation of Criteria for Detection of Antibody against Schistosoma japonicum—Enzyme-linked Immunosorbent Assay [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(6): 798-800. |
| [6] | TANG Xian-shi, JI Wen-xiang, XIONG Chun-rong, ZHOU Yong-hua, XU Yong-liang, TONG De-sheng. Study on anxiety-like behavior of mice with late-stage infection of Schistosoma japonicum [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(5): 622-628. |
| [7] | WANG Ji-peng. Research progress of stem cells in driving schistosome growth and development [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(4): 436-440. |
| [8] | LIANG Le, ZHANG Jing, SHEN Yu-juan, HU Yuan, CAO Jian-ping. Cyclic guanosine monophosphate-adenosine monophosphate promotes liver egg granuloma formation and fibrosis in mice infected with Schistosoma japonicum [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(4): 441-445. |
| [9] | ZHUO Yi-cheng, YANG Hai-cheng, LIU Cheng-hao, ZHANG Bao-cai, DUO Xiao-yong, ZHANG Shi-jie. Effect of osteopontin expression level on the growth and development of Echinococcus multilocularis protoscoleces [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(3): 299-304. |
| [10] | GAO Yuan, ZHANG Xiao-cheng, HU Yuan, CAO Jian-ping. Study on the inhibitory effect of natural killer cells on liver fibrosis of schistosomiasis [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(2): 168-174. |
| [11] | HONG Yang, LIN Jiao-jiao. Research progress on proteomics in Schistosoma japonicum [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(6): 725-730. |
| [12] | HUANG Ai-long, ZHANG Bei, SHEN Han-yu, CHEN Guo, LI Jing, ZHU Dan-dan, DUAN Yi-nong. Expression and function of triggering receptor expressed on myeloid cells 1 in the liver of mice infected with Schistosoma japonicum [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(5): 621-626. |
| [13] | ZHAO Cheng-si, QIN Min, TAN Ming-juan, MIAO Ting-ting, SHAO Tian-ye, LIU Xin-jian, WANG Yong. Effect of praziquantel on impaired renal function in mice with acute infection of Schistosoma japonicum [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(2): 200-209. |
| [14] | MO Xiao-jin, WU Qun-feng, FENG Zheng, XU Bin, ZHANG Ting, CHEN Shen-bo, HU Wei. Sexing Schistosoma japonicum cercariae by sequence characterized amplified region-PCR [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2020, 38(6): 777-780. |
| [15] | WANG Wei, ZHAO Cheng-si, MIAO Ting-ting, ZHOU Chun-lei, ZHANG Cheng-cheng, QIN Min, SHAO Tian-ye, WANG Yong. Praziquantel inhibits splenic macrophage proliferation and inflammatory reaction in mice infected with Schistosoma japonicum [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2020, 38(3): 263-270. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||