
CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES ›› 2022, Vol. 40 ›› Issue (4): 441-445.doi: 10.12140/j.issn.1000-7423.2022.04.004
• ORIGINAL ARTICLES • Previous Articles Next Articles
LIANG Le1,2( ), ZHANG Jing1, SHEN Yu-juan1, HU Yuan1, CAO Jian-ping1,*(
), ZHANG Jing1, SHEN Yu-juan1, HU Yuan1, CAO Jian-ping1,*( )
)
Received:2022-05-18
Revised:2022-06-11
Online:2022-08-30
Published:2022-09-07
Contact:
CAO Jian-ping
E-mail:lianglecdc@163.com;caojp@chinacdc.cn
Supported by:CLC Number:
LIANG Le, ZHANG Jing, SHEN Yu-juan, HU Yuan, CAO Jian-ping. Cyclic guanosine monophosphate-adenosine monophosphate promotes liver egg granuloma formation and fibrosis in mice infected with Schistosoma japonicum[J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(4): 441-445.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.jsczz.cn/EN/10.12140/j.issn.1000-7423.2022.04.004
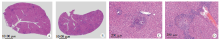
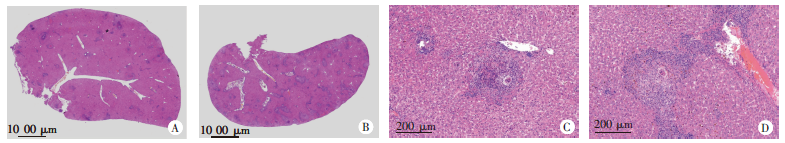
Fig. 1
cGAMP promotes liver granuloma formation in mice infected with S. japonicum(HE staining) A: Infected control group, showing the tissue structure damage and infiltration of inflammatory cells in the liver(× 25); B: Infected cGAMP-treated group, showing more severe tissue structure damage and robust infiltration of inflammatory cells in the liver(× 25); C: Infected control group, showing egg-induced granulomas in the livers of infected mice(× 200); D: Infected cGAMP-treated group, showing larger egg-induced granulomas in the livers of infected mice(× 200).
| [1] | Lin JJ. Endemic status and control of animal schistosomiasis in China[J]. Chin J Schisto Control, 2019, 31(1): 40-46. (in Chinese) |
| ( 林矫矫. 我国家畜血吸虫病流行情况及防控进展[J]. 中国血吸虫病防治杂志, 2019, 31(1): 40-46.) | |
| [2] |
Gryseels B, Polman K, Clerinx J, et al. Human schistosomiasis[J]. Lancet, 2006, 368(9541): 1106-1118.
pmid: 16997665 |
| [3] |
Colley DG, Secor WE. Immunology of human schistosomiasis[J]. Parasite Immunol, 2014, 36(8): 347-357.
doi: 10.1111/pim.12087 pmid: 25142505 |
| [4] |
Kamdem SD, Moyou-Somo R, Brombacher F, et al. Host regulators of liver fibrosis during human schistosomiasis[J]. Front Immunol, 2018, 9: 2781.
doi: 10.3389/fimmu.2018.02781 |
| [5] |
Wahl SM, Frazier-Jessen M, Jin WW, et al. Cytokine regulation of schistosome-induced granuloma and fibrosis[J]. Kidney Int, 1997, 51(5): 1370-1375.
pmid: 9150446 |
| [6] |
Medzhitov R. Recognition of microorganisms and activation of the immune response[J]. Nature, 2007, 449(7164): 819-826.
doi: 10.1038/nature06246 |
| [7] |
Gong W, Huang F, Sun L, et al. Toll-like receptor-2 regulates macrophage polarization induced by excretory-secretory antigens from Schistosoma japonicum eggs and promotes liver pathology in murine schistosomiasis[J]. PLoS Negl Trop Dis, 2018, 12(12): e0007000.
doi: 10.1371/journal.pntd.0007000 |
| [8] |
Xu Z, Xu L, Li W, et al. Innate scavenger receptor-a regulates adaptive T helper cell responses to pathogen infection[J]. Nat Commun, 2017, 8: 16035.
doi: 10.1038/ncomms16035 |
| [9] |
Paveley RA, Aynsley SA, Turner JD, et al. The mannose receptor (CD206) is an important pattern recognition receptor (PRR) in the detection of the infective stage of the helminth Schistosoma mansoni and modulates IFNgamma production[J]. Int J Parasitol, 2011, 41(13-14): 1335-1345.
doi: 10.1016/j.ijpara.2011.08.005 pmid: 22036898 |
| [10] |
Sun L, Wu J, Du F, et al. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the typeⅠinterferon pathway[J]. Science, 2013, 339(6121): 786-791.
doi: 10.1126/science.1232458 |
| [11] |
Li T, Chen ZJ. The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer[J]. J Exp Med, 2018, 215(5): 1287-1299.
doi: 10.1084/jem.20180139 |
| [12] |
Ablasser A, Gulen MF. The role of cGAS in innate immunity and beyond[J]. J Mol Med (Berl), 2016, 94(10): 1085-1093.
doi: 10.1007/s00109-016-1423-2 pmid: 27154323 |
| [13] |
Majumdar T, Chattopadhyay S, Ozhegov E, et al. Induction of interferon-stimulated genes by IRF3 promotes replication of Toxoplasma gondii[J]. PLoS Pathog, 2015, 11(3): e1004779.
doi: 10.1371/journal.ppat.1004779 |
| [14] |
Gallego-Marin C, Schrum JE, Andrade WA, et al. Cyclic GMP-AMP synthase is the cytosolic sensor of Plasmodium falciparum genomic DNA and activates typeⅠIFN in malaria[J]. J Immunol, 2018, 200(2): 768-774.
doi: 10.4049/jimmunol.1701048 pmid: 29212905 |
| [15] |
Hahn WO, Butler NS, Lindner SE, et al. cGAS-mediated control of blood-stage malaria promotes Plasmodium-specific germinal cen ter responses[J]. JCI Insight, 2018, 3(2): e94142.
doi: 10.1172/jci.insight.94142 |
| [16] |
Cai X, Chiu YH, Chen ZJ. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling[J]. Mol Cell, 2014, 54(2): 289-296.
doi: 10.1016/j.molcel.2014.03.040 |
| [17] |
Liang L, Shen Y, Hu Y, et al. cGAS exacerbates Schistosoma japonicum infection in a STING-typeⅠIFN-dependent and independent manner[J]. PLoS Pathog, 2022, 18(2): e1010233.
doi: 10.1371/journal.ppat.1010233 |
| [18] |
Decout A, Katz JD, Venkatraman S, et al. The cGAS-STING pathway as a therapeutic target in inflammatory diseases[J]. Nat Rev Immunol, 2021, 21(9): 548-569.
doi: 10.1038/s41577-021-00524-z |
| [19] |
Xu XD, Zhang DM, Sun W, et al. A Schistosoma japonicum chimeric protein with a novel adjuvant induced a polarized Th1 immune response and protection against liver egg burdens[J]. BMC Infect Dis, 2009, 9: 54.
doi: 10.1186/1471-2334-9-54 |
| [20] |
Ablasser A, Chen ZJ. cGAS in action: expanding roles in immunity and inflammation[J]. Science, 2019, 363(6431): eaat8657.
doi: 10.1126/science.aat8657 |
| [21] | Kwon J, Bakhoum SF. The cytosolic DNA-sensing cGAS-STING pathway in cancer[J]. Cancer Discov, 2020, 10(1): 6-39. |
| [22] | Yan H, Chen W. The promise and challenges of cyclic dinucleotides as molecular adjuvants for vaccine development[J]. Vaccines (Basel), 2021, 9(8): 917. |
| [23] |
Hou YJ, Lu H, Li JX, et al. A photoaffinity labeling strategy identified EF1A1 as a binding protein of cyclic dinucleotide 2′3′-cGAMP[J]. Cell Chem Biol, 2022, 29(1): 133-144.
doi: 10.1016/j.chembiol.2021.08.006 |
| [24] |
Elmanfi S, Yilmaz M, Ong WWS, et al. Bacterial cyclic dinucleotides and the cGAS-cGAMP-STING pathway: a role in periodontitis?[J]. Pathogens, 2021, 10(6): 675.
doi: 10.3390/pathogens10060675 |
| [25] |
Gursoy UK, Gursoy M, Kononen E, et al. Cyclic dinucleotides in oral bacteria and in oral biofilms[J]. Front Cell Infect Microbiol, 2017, 7: 273.
doi: 10.3389/fcimb.2017.00273 |
| [26] | Ahn J, Barber GN. STING signaling and host defense against microbial infection[J]. Exp Mol Med, 2019, 51(12): 1-10. |
| [1] | TAN Xiao, ZHU Qi, LIU Zhongqi, LI Jia, PENG Dingjin. Immunogenicity of Schistosoma japonicum Sj26gst mRNA vaccine candidate [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(5): 546-551. |
| [2] | LIU Huaman, Bikash Giri, FANG Chuantao, ZHENG Yameng, WU Huixin, ZENG Minhao, LI Shan, CHENG Guofeng. Identification of gender associated m6A modified circRNA in Schistosoma japonicum [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(5): 552-558. |
| [3] | LAN Weiming, XU Hui, XU Yin, QIU Tingting, XIE Shuying, DENG Fenglin, HU Shaoliang, LIU Huan, GUO Jiagang, ZENG Xiaojun. Study on early warning of high risk environment of Schistosoma japonicum infection by quantitative real-time PCR [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(4): 502-505. |
| [4] | WANG Xiao-ling, ZHANG Wei, YI Cun, CHEN Xiang-yu, YANG Wen-bin, XU Bin, HU Wei. The effect of SjGPR89 protein on the growth and development of Schistosoma japonicum [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(6): 701-707. |
| [5] | CHEN Guo, ZHU Dan-dan, DUAN Yi-nong. Research progress of immune regulation protein B7 family on immune regulation during Schistosoma japonicum infection [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(6): 774-779. |
| [6] | YAN Xiao-lan, WEN Li-yong, XIONG Yan-hong, ZHENG Bin, ZHANG Jian-feng, WANG Tian-ping, YU Li-ling, XU Guo-zhang, LIN Dan-dan, ZHOU Xiao-nong. Interpretation of Criteria for Detection of Antibody against Schistosoma japonicum—Enzyme-linked Immunosorbent Assay [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(6): 798-800. |
| [7] | TANG Xian-shi, JI Wen-xiang, XIONG Chun-rong, ZHOU Yong-hua, XU Yong-liang, TONG De-sheng. Study on anxiety-like behavior of mice with late-stage infection of Schistosoma japonicum [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(5): 622-628. |
| [8] | GAO Yuan, ZHANG Xiao-cheng, HU Yuan, CAO Jian-ping. Study on the inhibitory effect of natural killer cells on liver fibrosis of schistosomiasis [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(2): 168-174. |
| [9] | HONG Yang, LIN Jiao-jiao. Research progress on proteomics in Schistosoma japonicum [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(6): 725-730. |
| [10] | HUANG Ai-long, ZHANG Bei, SHEN Han-yu, CHEN Guo, LI Jing, ZHU Dan-dan, DUAN Yi-nong. Expression and function of triggering receptor expressed on myeloid cells 1 in the liver of mice infected with Schistosoma japonicum [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(5): 621-626. |
| [11] | ZHAO Cheng-si, QIN Min, TAN Ming-juan, MIAO Ting-ting, SHAO Tian-ye, LIU Xin-jian, WANG Yong. Effect of praziquantel on impaired renal function in mice with acute infection of Schistosoma japonicum [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(2): 200-209. |
| [12] | MO Xiao-jin, WU Qun-feng, FENG Zheng, XU Bin, ZHANG Ting, CHEN Shen-bo, HU Wei. Sexing Schistosoma japonicum cercariae by sequence characterized amplified region-PCR [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2020, 38(6): 777-780. |
| [13] | WANG Wei, ZHAO Cheng-si, MIAO Ting-ting, ZHOU Chun-lei, ZHANG Cheng-cheng, QIN Min, SHAO Tian-ye, WANG Yong. Praziquantel inhibits splenic macrophage proliferation and inflammatory reaction in mice infected with Schistosoma japonicum [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2020, 38(3): 263-270. |
| [14] | LI Zhi-dan, ZHANG Wei, WANG Xiao-ling, XU Bin, HU Wei. Effects of Schistosoma japonicum infection on OVA-induced allergic airway inflammation in mice [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2020, 38(3): 271-278. |
| [15] | TAN Xiao, XIAO Chu-li, XIAO Fei, WANG Shuo, QING Rui, HUANG Ze-zhi. IL-18 enhances the immunoprotective effect of pcDNA3.1/SjOST48 against Schistosoma japonicum infection [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2020, 38(3): 279-285. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||