
CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES ›› 2023, Vol. 41 ›› Issue (4): 404-411.doi: 10.12140/j.issn.1000-7423.2023.04.002
• ORIGINAL ARTICLES • Previous Articles Next Articles
DING Hongyun1( ), DONG Ying2,*(
), DONG Ying2,*( ), XU Yanchun2, DENG Yan2, LIU Yan2, WU Jing2, CHEN Mengni2, ZHANG Canglin2
), XU Yanchun2, DENG Yan2, LIU Yan2, WU Jing2, CHEN Mengni2, ZHANG Canglin2
Received:2022-10-13
Revised:2023-01-27
Online:2023-08-30
Published:2023-09-06
Contact:
*E-mail: Supported by:CLC Number:
DING Hongyun, DONG Ying, XU Yanchun, DENG Yan, LIU Yan, WU Jing, CHEN Mengni, ZHANG Canglin. Polymorphism analysis of multidrug resistance protein 1 gene in imported Plasmodium vivax in Yunnan Province[J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(4): 404-411.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.jsczz.cn/EN/10.12140/j.issn.1000-7423.2023.04.002
Table 1
Sequences of the primers used for amplifying the Pvmdr1 gene in P. vivax
| 片段 Fragment | 引物名称 Primer name | 引物序列(5'→3') Primer sequence (5'→3') | PCR轮次 PCR round | 产物长度/bp Product length/bp |
|---|---|---|---|---|
| F1 | MD-1F | TAACTCCTCACCGTTTGGGAAT | 第一轮 | 1 245 |
| MD-1R | TCATTGTTTGGTTGCTGGTTGC | First round | ||
| F2 | MD-2F | TTTATTACCATATTTACGTACGCAAG | 第一轮 | 1 385 |
| MD-2R | ATGATGATCGTAATTCTGTTTTCG | First round | ||
| F3 | MD-3F1 | CAACATCAAGTATAGTTTGTACAGC | 第一轮 | 1 368 |
| MD-3R1 | TGAACATCTCTGTTAATATGTGCTG | First round | ||
| MD-3F2 | TTAGTGTTTCGAAGAAGGTGCA | 第二轮 | 959 | |
| MD-3R2 | GTAGAGGGAGTACTTATTCGAGT | Second round | ||
| F4 | MD-4F | GCAGCATTTATAAGGACTCCG | 第一轮 | 1 387 |
| MD-4R | CTCATCACGGTAGATTTGCC | First round | ||
| F5 | MD-5F1 | GAGAAGGCTATTGATTATTCGAAT | 第一轮 | 1 571 |
| MD-5R1 | TTAACTATGTTTACTACGGTTAAGGG | First round | ||
| MD-5F2 | TCCTTCGAAAGGTACTACCCACT | 第二轮 | ||
| MD-5R2 | CATATGATCTGTGCACGTGCAT | Second round |
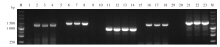
Fig. 1
Gel electrophoresis of PCR amplification products of Pvmdr1 genes in imported P. vivax in Yunnan Province M: DNA marker; 1, 5, 9, 15, 19: Negative control of the primary PCR; 10, 20: Negative control of the second round PCR; 2-4: Fragment F1; 6-8: Fragment F2; 11-14: The second round PCR product of fragment F3; 16-18: Fragment F4; 21-23: The second round PCR product of fragment F5.
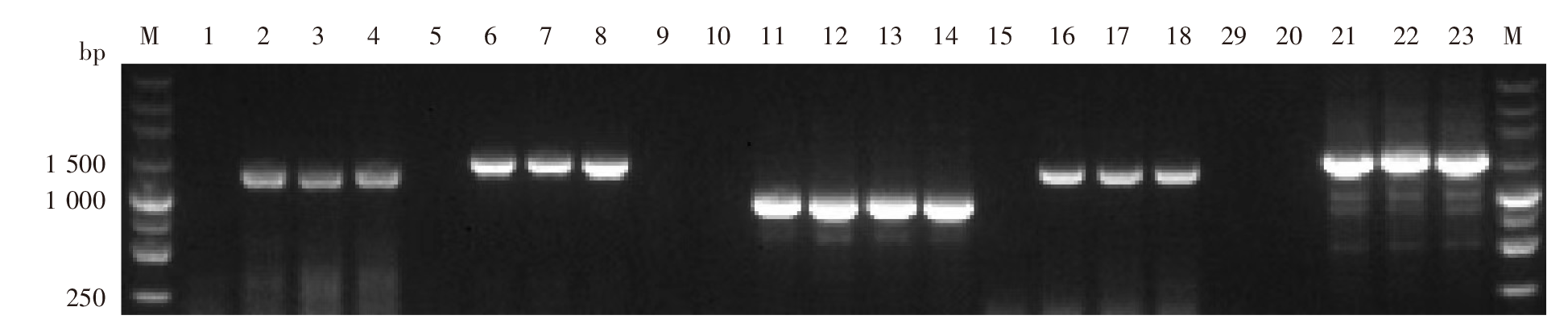
Table 3
Different haplotypes mutations in Pvmdr1 gene in imported P. vivax in Yunnan Province
| 序号 Order | 单倍型 Haplotype | 不同程度的多重突变型 Multiple mutants of different degrees | 单倍型数(检出率/%) No. haplotype (Detection rate/%) | ||||
|---|---|---|---|---|---|---|---|
| 类型 Type | 重性 Multiplicity | 2020 (n = 140) | 2021 (n = 119) | 合计 Total | |||
| 1 | Hap_24 | G698S/M908L/T958M/F1076L | 4 | 1(0.7) | - | 1(0.4) | |
| 2 | Hap_8 | T529T/G698S/M908L/T958M/F1076L | 5 | 29(20.7) | 19(16.0) | 48(18.5) | |
| 3 | Hap_9 | T529T/M908L/T958M/F1076L/K1393N | 5 | - | 4(3.4) | 4(1.5) | |
| 4 | Hap_21 | G698S/L845F/M908L/T958M/F1076L | 5 | 2(1.4) | 1(0.8) | 3(1.2) | |
| 5 | Hap_26 | K44K/G698S/M908L/T958M/F1076L | 5 | 1(0.7) | - | 1(0.4) | |
| 6 | Hap_27 | K44K/T529T/M908L/T958M/F1076L | 5 | 1(0.7) | - | 1(0.4) | |
| 7 | Hap_7 | S513R/T529T/G698S/M908L/T958M/K1393N | 6 | 6(4.3) | 4(3.4) | 10(3.9) | |
| 8 | Hap_4 | P8L/L310L/T529T/M908L/T958M/F1076L | 6 | 19(13.6) | 24(20.2) | 43(16.6) | |
| 9 | Hap_10 | S513R/T529T/G698S/M908L/T958M/S1358S | 6 | 21(15.0) | 4(3.4)a | 25(9.7) | |
| 10 | Hap_12 | T529T/G698S/M908L/T958M/F1076L/K1393N | 6 | - | 1(0.8) | 1(0.4) | |
| 11 | Hap_13 | S513R/T529T/G698S/M908L/T958M/F1076L | 6 | - | 1(0.8) | 1(0.4) | |
| 12 | Hap_16 | S513R/T529T/M908L/T958M/F1076L/S1358S | 6 | - | 1(0.8) | 1(0.4) | |
| 13 | Hap_18 | T529T/G698S/M908L/T958M/F1076L/S1358S | 6 | - | 1(0.8) | 1(0.4) | |
| 14 | Hap_20 | T409M/T529T/M908L/T958M/F1076L/K1393N | 6 | - | 1(0.8) | 1(0.4) | |
| 15 | Hap_23 | K44K/T529T/G698S/M908L/T958M/F1076L | 6 | - | 1(0.8) | 1(0.4) | |
| 16 | Hap_25 | T529T/G698S/A861E/M908L/T958M/F1076L | 6 | 1(0.7) | - | 1(0.4) | |
| 17 | Hap_2 | K44K/T529T/G698S/L845F/M908L/T958M/F1076L | 7 | 10(7.1) | 14(11.8) | 24(9.3) | |
| 18 | Hap_3 | P8L/L310L/T529T/G698S/M908L/T958M/F1076L | 7 | - | 1(0.8) | 1(0.4) | |
| 19 | Hap_5 | K44K/T529T/G698S/A861E/M908L/T958M/F1076L | 7 | 15(10.7) | 17(14.3) | 32(12.4) | |
| 20 | Hap_6 | K44K/T529T/G698S/M908L/T958M/F1076L/S1450L | 7 | 16(11.4) | 13(11.0) | 29(11.2) | |
| 21 | Hap_11 | K44K/T529T/G698S/M833I/M908L/T958M/F1076L | 7 | 3(2.1) | 3(2.5) | 6(2.3) | |
| 22 | Hap_14 | T409M/T529T/G698S/M908L/T958M/F1076L/K1393N | 7 | 8(5.7) | 4(3.4) | 12(4.6) | |
| 23 | Hap_17 | K44K/S513R/T529T/G698S/M908L/T958M/S1358S | 7 | - | 1(0.8) | 1(0.4) | |
| 24 | Hap_19 | S513R/T529T/M908L/T958M/L1022L/F1076L/K1355K | 7 | - | 1(0.8) | 1(0.4) | |
| 25 | Hap_22 | K44K/G698S/L845F/M908L/T958M/F1076L/L1120L/L1278L/S1450L | 9 | - | 1(0.8) | 1(0.4) | |
| 26 | Hap_15 | K44K/G520D/T529T/G698S/A861E/M908L/T958M/F1076L/I1348I/S1450L | 10 | 7(5.0) | 2(1.7) | 9(3.5) | |
| [1] | Xia ZG, Feng J, Zhang L, et al. Achieving malaria elimination in China: analysis on implementation and effectiveness of the surveillance-response system[J]. Chin J Parasitol Parasit Dis, 2021, 39(6): 733-741. (in Chinese) |
| (夏志贵, 丰俊, 张丽, 等. 中国消除疟疾: 监测响应系统的实施与成效分析[J]. 中国寄生虫学与寄生虫病杂志, 2021, 39(6): 733-741.) | |
| [2] | Zhang L, Feng J, Zhang SS, et al. Epidemiological characteristics of malaria in China, 2016[J]. Chin J Parasitol Parasit Dis, 2017, 35(6): 515-519. (in Chinese) |
| (张丽, 丰俊, 张少森, 等. 2016年全国疟疾疫情特征分析[J]. 中国寄生虫学与寄生虫病杂志, 2017, 35(6): 515-519.) | |
| [3] | Zhang L, Yi BY, Xia ZG, et al. Epidemiological characteristics of malaria in China, 2021[J]. Chin J Parasitol Parasit Dis, 2022, 40(2): 135-139. (in Chinese) |
| (张丽, 易博禹, 夏志贵, 等. 2021年全国疟疾疫情特征分析[J]. 中国寄生虫学与寄生虫病杂志, 2022, 40(2): 135-139.) | |
| [4] |
Rieckmann KH, Davis DR, Hutton DC. Plasmodium vivax resistance to chloroquine?[J]. Lancet, 1989, 334(8673): 1183-1184.
doi: 10.1016/S0140-6736(89)91792-3 |
| [5] |
(Myat-Phone-Kyaw, Myint-Oo, Myint-Lwin, et al. Emergence of chloroquine-resistant Plasmodium vivax in Myanmar (Burma)[J]. Trans R Soc Trop Med Hyg, 1993, 87(6): 687.
doi: 10.1016/0035-9203(93)90294-Z |
| [6] |
Singh RK. Emergence of chloroquine-resistant vivax malaria in South Bihar (India)[J]. Trans R Soc Trop Med Hyg, 2000, 94(3): 327.
doi: 10.1016/S0035-9203(00)90344-4 |
| [7] |
Phan GT, de Vries PJ, Tran BQ, et al. Artemisinin or chloroquine for blood stage Plasmodium vivax malaria in Vietnam[J]. Trop Med Int Health, 2002, 7(10): 858-864.
doi: 10.1046/j.1365-3156.2002.00948.x |
| [8] | Musset L, Heugas C, Naldjinan R, et al. Emergence of Plasmodium vivax resistance to chloroquine in French Guiana[J]. Antimicrob Agents Chemother, 2019, 63(11): e02116-e02118. |
| [9] |
Picot S, Brega S, Gérôme P, et al. Absence of nucleotide polymorphism in a Plasmodium vivax multidrug resistance gene after failure of mefloquine prophylaxis in French Guyana[J]. Trans R Soc Trop Med Hyg, 2005, 99(3): 234-237.
doi: 10.1016/j.trstmh.2004.09.007 |
| [10] |
Marfurt J, de Monbrison F, Brega S, et al. Molecular markers of in vivo Plasmodium vivax resistance to amodiaquine plus sulfadoxine-pyrimethamine: mutations in Pvdhfr and Pvmdr1[J]. J Infect Dis, 2008, 198(3): 409-417.
doi: 10.1086/589882 pmid: 18582193 |
| [11] |
Zeng WL, Zhao H, Zhao W, et al. Molecular surveillance and Ex vivo drug susceptibilities of Plasmodium vivax isolates from the China-Myanmar border[J]. Front Cell Infect Microbiol, 2021, 11: 738075.
doi: 10.3389/fcimb.2021.738075 |
| [12] |
Li JY, Zhang J, Li Q, et al. Ex vivo susceptibilities of Plasmodium vivax isolates from the China-Myanmar border to antimalarial drugs and association with polymorphisms in Pvmdr1 and Pvcrt-o genes[J]. PLoS Negl Trop Dis, 2020, 14(6): e0008255.
doi: 10.1371/journal.pntd.0008255 |
| [13] |
Xu SL, Zeng WL, Ngassa Mbenda HG, et al. Efficacy of directly-observed chloroquine-primaquine treatment for uncomplicated acute Plasmodium vivax malaria in northeast Myanmar: a prospective open-label efficacy trial[J]. Travel Med Infect Dis, 2020, 36: 101499.
doi: 10.1016/j.tmaid.2019.101499 |
| [14] |
Ratcliff A, Siswantoro H, Kenangalem E, et al. Therapeutic response of multidrug-resistant Plasmodium falciparum and P. vivax to chloroquine and sulfadoxine-pyrimethamine in southern Papua, Indonesia[J]. Trans R Soc Trop Med Hyg, 2007, 101(4): 351-359.
doi: 10.1016/j.trstmh.2006.06.008 |
| [15] |
Ganguly S, Saha P, Guha SK, et al. In vivo therapeutic efficacy of chloroquine alone or in combination with primaquine against vivax malaria in Kolkata, West Bengal, India, and polymorphism in Pvmdr1 and Pvcrt-o genes[J]. Antimicrob Agents Chemother, 2013, 57(3): 1246-1251.
doi: 10.1128/AAC.02050-12 |
| [16] |
Pukrittayakamee S, Imwong M, Looareesuwan S, et al. Therapeutic responses to antimalarial and antibacterial drugs in vivax malaria[J]. Acta Trop, 2004, 89(3): 351-356.
pmid: 14744561 |
| [17] |
Wang ZS, Wei CY, Pan YC, et al. Polymorphisms of potential drug resistant molecular markers in Plasmodium vivax from China-Myanmar border during 2008—2017[J]. Infect Dis Poverty, 2022, 11: 43.
doi: 10.1186/s40249-022-00964-2 |
| [18] | WHO. Malaria surveillance, monitoring & evaluation: a reference manua[M]. Geneva: WHO, 2018: 69-81. |
| [19] |
Chung DI, Jeong S, Dinzouna-Boutamba SD, et al. Evaluation of single nucleotide polymorphisms of Pvmdr1 and microsatellite genotype in Plasmodium vivax isolates from Republic of Korea military personnel[J]. Malar J, 2015, 14: 336.
doi: 10.1186/s12936-015-0845-6 |
| [20] |
Nomura T, Carlton JM, Baird JK, et al. Evidence for different mechanisms of chloroquine resistance in 2 Plasmodium species that cause human malaria[J]. J Infect Dis, 2001, 183(11): 1653-1661.
pmid: 11343215 |
| [21] |
Sá JM, Nomura T, Neves JD, et al. Plasmodium vivax: allele variants of the mdr1 gene do not associate with chloroquine resistance among isolates from Brazil, Papua, and monkey-adapted strains[J]. Exp Parasitol, 2005, 109(4): 256-259.
doi: 10.1016/j.exppara.2004.12.005 |
| [22] |
Carlton J. The Plasmodium vivax genome sequencing project[J]. Trends Parasitol, 2003, 19(5): 227-231.
pmid: 12763429 |
| [23] |
Walliker D, Quakyi IA, Wellems TE, et al. Genetic analysis of the human malaria parasite Plasmodium falciparum[J]. Science, 1987, 236(4809): 1661-1666.
pmid: 3299700 |
| [24] |
McConkey GA, Waters AP, McCutchan TF. The generation of genetic diversity in malaria parasites[J]. Annu Rev Microbiol, 1990, 44: 479-498.
pmid: 2252391 |
| [25] |
Ngassa Mbenda HG, Wang ML, Guo J, et al. Evolution of the Plasmodium vivax multidrug resistance 1 gene in the Greater Mekong Subregion during malaria elimination[J]. Parasit Vectors, 2020, 13(1): 67.
doi: 10.1186/s13071-020-3934-5 |
| [26] |
Kittichai V, Nguitragool W, Ngassa Mbenda HG, et al. Genetic diversity of the Plasmodium vivax multidrug resistance 1 gene in Thai parasite populations[J]. Infect Genet Evol, 2018, 64: 168-177.
doi: 10.1016/j.meegid.2018.06.027 |
| [27] |
Villena FE, Maguiña JL, Santolalla ML, et al. Molecular surveillance of the Plasmodium vivax multidrug resistance 1 gene in Peru between 2006 and 2015[J]. Malar J, 2020, 19(1): 450.
doi: 10.1186/s12936-020-03519-8 |
| [28] |
Cubides JR, Camargo-Ayala PA, Niño CH, et al. Simultaneous detection of Plasmodium vivax dhfr, dhps, mdr1 and crt-o resistance-associated mutations in the Colombian Amazonian region[J]. Malar J, 2018, 17(1): 130.
doi: 10.1186/s12936-018-2286-5 |
| [29] |
Huang F, Li SG, Tian P, et al. Genetic polymorphisms in genes associated with drug resistance in Plasmodium vivax parasites from northeastern Myanmar[J]. Malar J, 2022, 21(1): 66.
doi: 10.1186/s12936-022-04084-y |
| [30] |
Orjuela-Sánchez P, de Santana Filho FS, Machado-Lima A, et al. Analysis of single-nucleotide polymorphisms in the crt-o and mdr1 genes of Plasmodium vivax among chloroquine-resistant isolates from the Brazilian Amazon region[J]. Antimicrob Agents Chemother, 2009, 53(8): 3561-3564.
doi: 10.1128/AAC.00004-09 pmid: 19451296 |
| [31] |
Tacoli C, Gai PP, Siegert K, et al. Characterization of Plasmodium vivax Pvmdr1 polymorphisms in isolates from mangaluru, India[J]. Am J Trop Med Hyg, 2019, 101(2): 416-417.
doi: 10.4269/ajtmh.19-0224 |
| [32] |
Diez Benavente E, Ward Z, Chan W, et al. Genomic variation in Plasmodium vivax malaria reveals regions under selective pressure[J]. PLoS One, 2017, 12(5): e0177134.
doi: 10.1371/journal.pone.0177134 |
| [33] |
Imwong M, Pukrittayakamee S, Pongtavornpinyo W, et al. Gene amplification of the multidrug resistance 1 gene of Plasmodium vivax isolates from Thailand, Laos, and Myanmar[J]. Antimicrob Agents Chemother, 2008, 52(7): 2657-2659.
doi: 10.1128/AAC.01459-07 |
| [34] |
González-Cerón L, Montoya A, Corzo-Gómez JC, et al. Genetic diversity and natural selection of Plasmodium vivax multi-drug resistant gene (Pvmdr1) in Mesoamerica[J]. Malar J, 2017, 16(1): 261.
doi: 10.1186/s12936-017-1905-x |
| [35] |
Zhao Y, Wang L, Soe MT, et al. Molecular surveillance for drug resistance markers in Plasmodium vivax isolates from symptomatic and asymptomatic infections at the China-Myanmar border[J]. Malar J, 2020, 19(1): 281.
doi: 10.1186/s12936-020-03354-x |
| [36] |
Lu F, Lim CS, Nam DH, et al. Genetic polymorphism in Pvmdr1 and Pvcrt-o genes in relation to in vitro drug susceptibility of Plasmodium vivax isolates from malaria-endemic countries[J]. Acta Trop, 2011, 117(2): 69-75.
doi: 10.1016/j.actatropica.2010.08.011 |
| [37] |
Barnadas C, Ratsimbasoa A, Tichit M, et al. Plasmodium vivax resistance to chloroquine in Madagascar: clinical efficacy and polymorphisms in Pvmdr1 and Pvcrt-o genes[J]. Antimicrob Agents Chemother, 2008, 52(12): 4233-4240.
doi: 10.1128/AAC.00578-08 pmid: 18809933 |
| [1] | LI Benfu, WANG Zhengqing, XU Qian, ZI Jinrong, YAN Xinliu, PENG Jia, LI Jianxiong, CAI Xuan, WU Fangwei, YANG Yaming. Sequence analysis of mitochondrial co1 and nd1 genes in Echinococcus granulosus in Yunnan Province [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(3): 306-311. |
| [2] | WANG Guan-xi, LI Ya-shu, LI Yue-yue, CAO Yuan-yuan, YANG Meng-meng, ZHANG Mei-hua, WU Jing-yao, LIANG Cheng, LI Ju-lin, ZHOU Hua-yun, TANG Jian-xia, ZHU Guo-ding. Resistance to deltamethrin and knockdown resistance mutation in Aedes albopictus from Jiangsu Province [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(4): 468-474. |
| [3] | SHI Tian-qi, CHEN Jun-hu. Research progress on reticulocyte binding proteins associated with Plasmodium vivax invasion of reticulocytes [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(3): 396-401. |
| [4] | PU Li-hua, ZHANG Xing-ze, GUAN Shao-jun, CHENG Wen-jie, ZOU Feng-cai, MAO Hua-ming, YANG Jian-fa. Blastocystis sp. infection in dairy cattle in Yunnan Province and its gene subtype analysis [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(6): 809-815. |
| [5] | CAI Xuan, YANG Ya-ming, LI Ben-fu, YAN Xin-liu, PENG Jia, ZI Jin-rong, WU Fang-wei. Investigation on the prevalence of human parasitic infections in the ecoregion of southern part of Yunnan-Guangxi-Guangdong neighboring area, Yunnan Province in 2015 [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(6): 848-852. |
| [6] | ZI Jin-rong, WANG Li-bo, YANG Ya-ming, LI Ben-fu, YAN Xin-liu, PENG Jia, CAI Xuan, WANG Zheng-qing, DU Zun-wei, WU Fang-wei. Current status of Ascaris lumbricoides infection in populations in Yunan Province, 2015 [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(2): 273-277. |
| [7] | WU Fang-wei, WANG Li-bo, LI Ben-fu, YAN Xin-liu, ZI Jin-rong, PENG Jia, CAI Xuan, BAO Xue-ying, YANG Ya-ming. Survey on current status of human hookworm infection in Yunnan Province in 2015 [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2020, 38(6): 781-784. |
| [8] | JIN Hang-yi, ZHANG Ling-ling, ZHU Su-juan, XU Wei-min, CHEN Jun-fang, RUAN Wei, YAO Li-nong, CHEN Hua-liang. Analysis of genetic polymorphisms of merozoite surface protein-1 and circumsporozoite protein of Plasmodium vivax [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2020, 38(3): 323-331. |
| [9] | Shu-ping LIU, Ying DONG, Yan-chun XU, Yan LIU, Yan DENG, Cang-lin ZHANG, Meng-ni CHEN. The mutation polymorphism of G6PD gene coding region in vivax malaria patients in Yunnan Province, China [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2020, 38(2): 146-151. |
| [10] | Li ZHANG, Jun FENG, Hong TU, Zhi-gui XIA, Shui-sen ZHOU. Challenges in malaria elimination: the epidemiological characteristics of Plasmodium vivax in China from 2011 to 2018 [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2019, 37(5): 532-538. |
| [11] | Ying DONG, Shu-ping LIU, Yan-chun XU, Yan LIU, Yan DENG, Meng-ni CHEN. Mutations and predicted structure change of G6PD isolated from a patient with primaquine-induced hemolysis in Yunnan Province [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2019, 37(4): 399-405. |
| [12] | Rui YANG, Yu-ting ZHENG, Xiao-yu YANG, Li-min DONG, Zu-rui LIN, Yao-wu ZHOU, Xu-can ZENG, Hong-bin LI, Jin-yong JIANG. Investigation on malaria vectors in Jinghong, a border area in Yunnan Province [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2019, 37(4): 406-410. |
| [13] | Cang-lin ZHANG, Xue-ying BAO, Jia PENG, Jin-rong ZI, Zhen RAN, Na LU, Ya-ming YANG. Species identification of Pomacea snails in southwest Yunan Province based on COⅠgene polymorphism [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2019, 37(1): 75-81. |
| [14] | DONG Ying1, DENG Yan1, XU Yan-chun1, CHEN Meng-ni1, MAO Xiang-hua1, . Analysis of gene sequence polymorphisms and prediction of antigen epitopes of merozoite surface protein-3 in Plasmodium falciparum from different infection sources [J]. , 2018, 36(3): 3-210-217. |
| [15] | Cheng-yun YANG, Su-hua LI, Ya-lan ZHANG, Rui-min ZHOU, Ying LIU, Dan QIAN, Yu-ling ZHAO, Bian-li XU, Hong-wei ZHANG, Yan DENG. Analysis of mutations of Plasmodium falciparum multidrug resistance gene 1 and K13 gene in imported Plasmodium falciparum in Henan Province [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2018, 36(2): 97-102. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||