
CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES ›› 2020, Vol. 38 ›› Issue (5): 580-588.doi: 10.12140/j.issn.1000-7423.2020.05.009
• ORIGGIINAL ARTNICLES • Previous Articles Next Articles
ZHANG Cang-lin1( ), NIE Ren-hua1, XU Dan2, LV Gao-wei3, WANG Jian4, YANG Ya-ming1, DENG Yan1, LIU Yan1, ZHOU Hong-ning1,*(
), NIE Ren-hua1, XU Dan2, LV Gao-wei3, WANG Jian4, YANG Ya-ming1, DENG Yan1, LIU Yan1, ZHOU Hong-ning1,*( )
)
Received:2020-04-14
Online:2020-10-30
Published:2020-11-12
Contact:
ZHOU Hong-ning
E-mail:43773923@qq.com;zhouhn66@163.com
Supported by:CLC Number:
ZHANG Cang-lin, NIE Ren-hua, XU Dan, LV Gao-wei, WANG Jian, YANG Ya-ming, DENG Yan, LIU Yan, ZHOU Hong-ning. Correlation of Pfcrt, Pfmdr and PfK13 gene polymorphisms and in vitro drug susceptibility of Plasmodium falciparum isolates from China-Myanmar border region[J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2020, 38(5): 580-588.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.jsczz.cn/EN/10.12140/j.issn.1000-7423.2020.05.009
Table 2
Sequences of the primers used to amplify the Pfcrt, Pfmdr and PfK13 genes of P. falciparum isolates
| 基因Gene | 引物Primer | 引物序列(5′→3′) Primer sequence (5′→3′) | PCR轮次 PCR round | 产物片段/bp Amplicon size/bp |
|---|---|---|---|---|
| Pfcrt | 76A | GCGCGCGCATGGCTCACGTTTAGGTGGAG | 第1轮 | 206 |
| 76B | GGGCCCGGCGGATGTTACAAAACTATAGTTACC | |||
| 76D1 | TGTGCTCATGTGTTTAAACTT | 第2轮 | 145 | |
| 76D2 | CAAAACTATAGTTACCAATTTTG | |||
| Pfmdr | 86A | GCGCGCGTTGAACAAAAAGAGTACCGCTG | 第1轮 | 450 |
| 86B | GGGCCCTCGTACCAATTCCTGAACTCAC | |||
| 86D1 | TTTACCGTTTAAATGTTTACCTGC | 第2轮 | 291 | |
| 86D2 | CCATCTTGATAAAAAACACTTCTT | |||
| 1246A | GGGGGATGACAAATTTTCAAGATTA | 第1轮 | 295 | |
| 1246B | GGGGGACTAACACGTTTAACATCTT | |||
| 1246D1 | AATGTAAATGAATTTTCAAACC | 第2轮 | 203 | |
| 1246D2 | CATCTTCTCTTCCAAATTTGATA | |||
| PfK13 | PCR_F | CGGAGTGACCAAATCTGGGA | 第1轮 | 2 181 |
| PCR_R | GGGAATCTGGTGGTAACAGC | |||
| N1_F | GCCAAGCTGCCATTCATTTG | 第2轮 | 849 | |
| N1_R | GCCTTGTTGAAAGAAGCAGA |
Table 3
In vitro sensitivity of P. falciparum and cross resistance of P. falciparum in China-Myanmar Border Region in 2009
| 药物 Drug | 测定血样 No. sample tested | 抗性率/% Resistance rate/% | 平均抑制浓度a/nmol·L-1 Mean inhibition concentrationa/nmol·L-1 | ID50 (95%可信限) ID50 (95%CI) | 对其他抗疟药的抗性血样(交叉抗性率/%) No. resistance to other antimalarials (Proportion of cross resistance/%) | |||
|---|---|---|---|---|---|---|---|---|
| 青蒿素 Artemisinin | 氯喹 Chloroquine | 哌喹 Piperaquine | 咯萘啶 Pyronaridine | |||||
| 青蒿素 Artemisinin | 42 | 40.5 | 640 | 84.8 (44.2~ 162.7) | - | 15(88.2) | 3(17.6) | 13(76.5) |
| 氯喹[ | 42 | 95.2 | 880 | 320.5 (57.9~1775) | 15(37.5) | - | 3(7.5) | 21(52.5) |
| 哌喹[ | 42 | 7.1 | 440 | 128.2 (125.1~ 131.4) | 3(100) | 3(100) | - | 3(100) |
| 咯萘啶[ | 42 | 54.8 | 410 | 96.0 (85.4~ 107.9) | 13(56.5) | 21(91.3) | 3(13.0) | - |

Fig. 1
Results of gel electrophoresis of PCR amplification product of Pfcrt, Pfmdr and PfK13 genes from P. falciparum isolates in China-Myanmar Border Region in 2009 A: Pfmdr gene mutant 86; B: Pfmdr gene mutant 1246; C: Pfcrt gene; D: PfK13 gene. 1-8: P. falciparum isolates; M: DNA marker; N1: Blank control of nested-PCR first cycle; N2: Blank control of nested-PCR second cycle
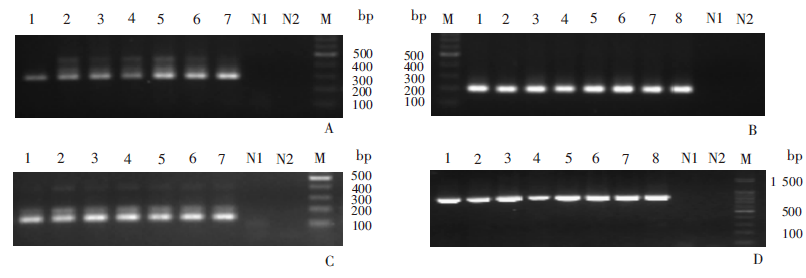
Table 4
The frequency distribution of different genotypes of Pfcrt, Pfmdr and PfK13 in in vitro drug susceptibility test of P. falciparum isolates from China-Myanmar Border Region in 2009
| 基因类型a Genotypea | 血样数量 No. isolates | 百分比/% Percentage/% | 体外检测对各药物产生抗性的血样数 Cases of in vitro resistance to drugs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 青蒿素 Artemisinin | 氯喹 Chloroquine | 哌喹 Piperaquine | 咯萘啶 Pyronaridine | ||||||
| Pfcrt基因 (体外,n = 35) Pfcrt gene (in vitro, n = 35) | |||||||||
| 三重基因突变C72V73I74E75T76 Triple-mutant genotype C72V73I74E75T76 | 37 | 100 | 12 | 28 | 3 | 16 | |||
| Pfmdr基因(体外,86位点n = 45,1246位点n = 47) Pfmdr gene (in vitro, n = 45 at codon 86, n = 47 at codon 1246) | |||||||||
| 单基因突变Y1246 Single-mutant genotype Y1246 | 2 | 4.1 | 0 | 2 | 0 | 1 | |||
| 单基因突变N86Y1246 Single-mutant genotype N86Y1246 | 46 | 93.9 | 16 | 34 | 3 | 21 | |||
| 双重突变Y86Y1246 Double-mutant genotype Y86Y1246 | 1 | 2.0 | 0 | 1 | 0 | 0 | |||
| PfK13基因(体外, n = 20) PfK13 gene (in vitro, n = 20) | |||||||||
| 野生型F446S492C580 Wild-type genotype F446S492C580 | 8 | 40.0 | 3 | 5 | 2 | 3 | |||
| 单基因突变I446S492C580 Single-mutant genotype I446S492C580 | 10 | 50.0 | 4 | 9 | 1 | 8 | |||
| 单基因突变F446L/S492C580 Single-mutant genotype F446L/S492C580 | 1 | 5.0 | 1 | 1 | 0 | 1 | |||
| 单基因突变F446S492Y580 Single-mutant genotype F446S492Y580 | 1 | 5.0 | 1 | 1 | 0 | 1 | |||
| Pfcrt/Pfmdr 多基因突变I74E75T76/Y1246 Pfcrt/Pfmdr multi-mutant genotypes I74E75T76/Y1246 | 29 | 69.0 | 8 | 21 | 2 | 10 | |||
| Pfcrt/Pfmdr多基因突变I74E75T76/Y86Y1246 Pfcrt/Pfmdr multi-mutant genotypes I74E75T76/Y86Y1246 | 1 | 2.4 | 0 | 1 | 0 | 0 | |||
| Pfcrt/Pfmdr/PfK13多基因突变I74E75T76/Y1246/I446 Pfcrt/Pfmdr/PfK13 multi-mutant genotypes I74E75T76/Y1246/I446 | 6 | 14.3 | 3 | 5 | 1 | 5 | |||
| Pfcrt/Pfmdr/PfK13多基因突变I74E75T76/Y1246/L/S492 Pfcrt/Pfmdr/PfK13 multi-mutant genotypes I74E75T76/Y1246/L/S492 | 1 | 2.4 | 1 | 1 | 0 | 1 | |||
| Pfmdr/PfK13多基因突变Y1246/I446 Pfmdr/PfK13 multi-mutant genotypes Y1246/I446 | 4 | 9.5 | 1 | 4 | 0 | 3 | |||
| Pfmdr/PfK13多基因突变Y1246/Y580 Pfmdr/ PfK13 multi-mutant genotypes Y1246/Y580 | 1 | 2.4 | 1 | 1 | 0 | 1 | |||
| [1] | WHO. WHO World Malaria Report 2019[R]. Geneva: WHO, 2019. |
| [2] | Guan YX, Tang LH. Molecular markers related to antimalarial resistance of Plasmodium falciparum and their applications on the surveillance to drug resistance[J]. Chin J Parasitol Parasit Dis, 2005,23(2):117-120. (in Chinese) |
| ( 官亚宜, 汤林华. 恶性疟原虫药物抗性相关的分子标记及其在药物抗性监测中的应用[J]. 中国寄生虫学与寄生虫病杂志, 2005,23(2):117-120.) | |
| [3] |
Harinasuta T, Suntharasamai P, Viravan C. Chloroquine-resistant falciparum malaria in Thailand[J]. Lancet, 1965,2(7414):657-660.
doi: 10.1016/s0140-6736(65)90395-8 pmid: 4158213 |
| [4] | Marsh K. Malaria disaster in Africa[J]. Lancet, 1998,352(9132):924. |
| [5] | Liu DQ, Cai XZ. The situation of sensitivity of Plasmodium falciparum to antimalarial drugs in China[J]. Chin J Parasitol Parasit Dis, 1996,14(1):37-41. (in Chinese) |
| ( 刘德全, 蔡贤铮. 我国恶性疟原虫对抗疟药敏感性的现状[J]. 中国寄生虫学与寄生虫病杂志, 1996,14(1):37-41.) | |
| [6] | Che LG, Huang KG, Yang HL. The sensitivity of pyronaridine to chloroquine-resistant Plasmodium falciparum strain[J]. Chin J Parasitol Parasit Dis, 1984,2(4):280-280. (in Chinese) |
| ( 车立刚, 黄开国, 杨恒林. 抗氯喹恶性疟原虫株对咯萘啶敏感性的测定[J]. 中国寄生虫学与寄生虫病杂志, 1984,2(4):280-280.) | |
| [7] | Liu GZ, Chen LG, Huang KG, et al. A suvery on the susceptibility of falciparum malaria to chloroquine in the desert sand area of Xinping county in Yunnan Province[J]. Chin J Parasitol Parasit Dis, 1983,1(4):6-7. (in Chinese) |
| ( 刘国志, 车立刚, 黄开国, 等. 云南新平县漠沙地区恶性疟对氯喹敏感性的调查[J]. 中国寄生虫学与寄生虫病杂志, 1983,1(4):6-7.) | |
| [8] | Liu DQ, Feng XP, Yang HL, et al. Fluctuation in the resistance of Plasmodium falciparum to chloroquine in China[J]. Chin J Parasitol Parasit Dis, 2005,23(1):7-31. (in Chinese) |
| ( 刘德全, 冯晓平, 杨恒林, 等. 我国恶性疟原虫对氯喹抗性的消长[J]. 中国寄生虫学与寄生虫病杂志, 2005,23(1):7-31.) | |
| [9] | Yang HL, Liu DQ, Dong Y, et al. The sensitivity of in vitro of Plasmodium falciparum to seven antimalarial drugs[J]. Chin J Parasitol Parasit Dis, 1995,13(2):111-113. (in Chinese) |
| ( 杨恒林, 刘德全, 董莹, 等. 体外测定恶性疟原虫对七种抗疟药的敏感性[J]. 中国寄生虫学与寄生虫病杂志, 1995,13(2):111-113.) | |
| [10] | Yang YM, Yang HL, Dong Y, et al. The sensitivity of in vitro of Plasmodium falciparum to seven antimalarial drugs in China-Myanmar border areas[J]. J Practic Parasit Dis, 1994, ( 3):23-26. (in Chinese) |
| ( 杨亚明, 杨恒林, 董莹, 等. 中缅边境地区恶性疟原虫对7种抗疟药敏感性的体外测定[J]. 实用寄生虫病杂志, 1994, ( 3):23-26.) | |
| [11] | Yang HL, Yang PF, Yang YM. The susceptibility of in vitro of Plasmodium falciparum to mefloquine, quinine, ampicolaquine, chloroquine, sulfadioxine/pyrimethamine and clonaphthalidine in southern Yunnan[J]. Chin J Parasitol Parasit Dis, 1994,12(2):140-142. (in Chinese) |
| ( 杨恒林, 杨品芳, 杨亚明. 云南南部恶性疟原虫对甲氟喹、奎宁、氨酚喹、氯喹、磺胺多辛/乙胺嘧啶及咯萘啶敏感性的体外测定[J]. 中国寄生虫学与寄生虫病杂志, 1994,12(2):140-142.) | |
| [12] | Huang QL, Zhou QL, Wu Z, et al. A study on the sensitivity of Plasmodium falciparum to chloroquine, qupiperaquine, ampicolaquine, mefloquine and quinine in Hainan Province[J]. Chin J Parasitol Parasit Dis, 1988,6(s1):19. (in Chinese) |
| ( 黄祺林, 周洁娴, 伍柱, 等. 海南省恶性疟原虫对氯喹、喹哌、氨酚喹、甲氟喹及奎宁敏感性的继续观察[J]. 中国寄生虫学与寄生虫病杂志, 1988,6(s1):19.) | |
| [13] | WHO. Guidelines for the treatment of malaria[S]. Geneva: WHO, 2006. |
| [14] | Song J, Jiang GF, Chen PQ, et al. Analysis of Pfcrt polymorphism of chloroquine-resistant gene of Plasmodium falciparum in Hainan Province[J]. Chin J Parasit Dis Control, 2005,18(3):175-177. (in Chinese) |
| ( 宋杰, 江钢锋, 陈沛泉, 等. 海南省恶性疟原虫氯喹抗性相关基因Pfcrt多态性分析[J]. 中国寄生虫病防治杂志, 2005,18(3):175-177.) | |
| [15] |
Babiker HA, Pringle SJ, Abdel-Muhsin A, et al. High-level chloroquine resistance in Sudanese isolates of Plasmodium falciparum is associated with mutations in the chloroquine resistance transporter gene Pfcrt and the multidrug resistance gene Pfmdr1[J]. J Infect Dis, 2001,183(10):1535-1538.
doi: 10.1086/320195 pmid: 11319692 |
| [16] |
Djimde A, Doumbo OK, Cortese JF, et al. A molecular marker for chloroquine-resistant falciparum malaria[J]. N Engl J Med, 2001,344(4):257-263.
doi: 10.1056/NEJM200101253440403 pmid: 11172152 |
| [17] |
Lakshmanan V, Bray PG, Verdier-Pinard D, et al. A critical role for PfCRT K76T in Plasmodium falciparum verapamil-reversible chloroquine resistance[J]. EMBO J, 24(13):2294-2305.
doi: 10.1038/sj.emboj.7600681 pmid: 15944738 |
| [18] | WHO. Status report on artemisinin resistance and ACT efficacy[R]. Geneva: WHO, 2018. |
| [19] |
Imwong M, Suwannasin K, Kunasol C, et al. The spread of artemisinin-resistant Plasmodium falciparum in the Greater Mekong subregion: a molecular epidemiology observational study[J]. Lancet Infect Dis, 2017,17:491-497.
doi: 10.1016/S1473-3099(17)30048-8 pmid: 28161569 |
| [20] |
Amato R, Lim P, Miotto O, Amaratunga C, et al. Genetic markers associated with dihydroartemisinin-piperaquine failure in Plasmodium falciparum malaria in Cambodia: a genotype-phenotype association study[J]. Lancet Infect Dis, 2017,17:164-173.
doi: 10.1016/S1473-3099(16)30409-1 pmid: 27818095 |
| [21] |
Witkowski B, Duru V, Khim N, et al. A surrogate marker of piperaquine-resistant Plasmodium falciparum malaria: a phenotype-genotype association study[J]. Lancet Infect Dis, 2017,17:174-183.
doi: 10.1016/S1473-3099(16)30415-7 pmid: 27818097 |
| [22] | Liu DQ, Ren DX. A medium for the determination of resistance to Plasmodium falciparum in study site[J]. Chin J Parasitol Parasit Dis, 1989,7(2):112-114. (in Chinese) |
| ( 刘德全, 任道性. 一种便于现场测定恶性疟原虫抗药性的培养基[J]. 中国寄生虫学与寄生虫病杂志, 1989,7(2):112-114.) | |
| [23] | Rieckmann KH, Campbell GH, Sax LJ, et al. Drug sensitivity of Plasmodium falciparum[J]. 1978,1(8054):22-23. |
| [24] | Lin SG, Liu DQ, Zhuo KR, et al. Determination of Plasmodium falciparum to antimalarials in Ledong County, Hainan Province[J]. J Trop Med, 2005,5(8):1707-1708. (in Chinese) |
| ( 林世干, 刘德全, 卓开仁, 等. 海南省乐东县恶性疟原虫对抗疟药的敏感性测定[J]. 中国热带医学, 2005,5(8):1707-1708.) | |
| [25] | Zhang YL, Pan WQ. Research progress on artemisinin resistance in Plasmodium falciparum[J]. Chin J Parasitol Parasit Dis, 2015,33(6):418-424. (in Chinese) |
| ( 张逸龙, 潘卫庆. 恶性疟原虫对青蒿素产生抗性的研究进展[J]. 中国寄生虫学与寄生虫病杂志, 2015,33(6):418-424. | |
| [26] | Zhang CL, Zhou HN, Nie RH, et al. Species identification and sequence analysis of Plasmodium spp. in border areas of Yunnan Province by 18S rRNA-based nested PCR[J]. Chin J Parasitol Parasit Dis, 2016,34(3):220-226. (in Chinese) |
| ( 张苍林, 周红宁, 聂仁华, 等. 云南省边境地区疟原虫18S rRNA基因种类鉴定与序列分析[J]. 中国寄生虫学与寄生虫病杂志, 2016,34(3):220-226.) | |
| [27] |
Ariey F, Witkowski B, Amaratunga C, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria[J]. Nature, 2014,505(7481):50-55.
doi: 10.1038/nature12876 pmid: 24352242 |
| [28] | Zhang CL, Zhou HN, Wan J, et al. In vitro sensitivity of Plasmodium falciparum isolates from China-Myanmar border region to chloroquine, piperaquine and pyronaridine[J]. Chin J Parasitol Parasit Dis, 2012,30(1):41-44. (in Chinese) |
| ( 张苍林, 周红宁, 王剑, 等. 中缅边境地区恶性疟原虫对氯喹、哌喹、咯萘啶敏感性的体外测定[J]. 中国寄生虫学与寄生虫病杂志, 2012,30(1):41-44.) | |
| [29] |
Yang HL, Liu DQ, Yang YM, et al. In vitro sensitivity of Plasmodium falciparum to eight antimalarials in China-Myanmar and China-Lao PDR border areas[J]. Southeast Asian J Trop Med Public Health, 1997,28(3):460-464.
pmid: 9561592 |
| [30] | Yang HL, Yang PF, Li XL, et al. The change of Plasmodium falciparum resistance to chloroquine in Yunnan Province, China[J]. J Pathog Biol, 2005,18(5):368-370. (in Chinese) |
| ( 杨恒林, 杨品芳, 李兴亮, 等. 云南省恶性疟原虫对氯喹抗性的变化[J]. 中国病原生物学杂志, 2005,18(5):368-370.) | |
| [31] |
Witkowski B, Amaratunga C, Khim N, et al. Novel phenotypic assays for the detection of artemisinin-resistant Plasmodium falciparum malaria in Cambodia: in vitro and ex vivo drug-response studies[J]. Lancet Infect Dis, 2013,13(12):1043-1049.
doi: 10.1016/S1473-3099(13)70252-4 pmid: 24035558 |
| [32] |
Andriantsoanirina V, Ratsimbasoa A, Bouchier C, et al. Chloroquine clinical failures in P. falciparum malaria are associated with mutant Pfmdr-1, not Pfcrt in Madagascar[J]. PLoS One, 2010,5(10):e13281.
doi: 10.1371/journal.pone.0013281 pmid: 20967251 |
| [33] |
Flueck TP, Jelinek T, Kilian AH, et al. Correlation of in vivo-resistance to chloroquine and allelic polymorphisms in Plasmodium falciparum isolates from Uganda[J]. Trop Med Int Health, 2000,5(3):174-178.
doi: 10.1046/j.1365-3156.2000.00543.x pmid: 10747279 |
| [34] |
Miotto O, Amato R, Ashley EA, et al. Genetic architecture of artemisinin-resistant Plasmodium falciparum[J]. Nat Genet, 2015,47(3):226-234.
doi: 10.1038/ng.3189 pmid: 25599401 |
| [35] |
Huang F, Takala-Harrison S, Jacob CG, et al. A single mutation in K13 predominates in Southern China and is associated with delayed clearance of Plasmodium falciparum following artemisinin treatment[J]. J Infect Dis, 2015,212(10):1629-1635.
doi: 10.1093/infdis/jiv249 pmid: 25910630 |
| [36] | Zhang GQ, Guan GQ, Hu L, et al. The polymorphisms of Pfcrt 72-76 in Plasmodium falciparum isolated from China and their relationships with chloroquine resistance[J]. Chin J Zoono, 2009,25(8):725-728. (in Chinese) |
| ( 张国庆, 官亚宜, 胡铃, 等. 中国恶性疟原虫氯喹抗药性相关基因Pfcrt 72-76位点基因型分析[J]. 中国人兽共患病学报, 2009,25(8):725-728.) | |
| [37] |
Yang Z, Zhang Z, Sun X, et al. Molecular analysis of chloroquine resistance in Plasmodium falciparum in Yunnan Province, China[J]. Trop Med Int Health, 2007,12(9):1051-1060.
doi: 10.1111/j.1365-3156.2007.01882.x pmid: 17875016 |
| [38] |
Hao M, Jia D, Li Q, et al. In vitro sensitivities of Plasmodium falciparum isolates from the China-Myanmar border to piperaquine and association with polymorphisms in candidate genes[J]. Antimicrob Agents Chemother, 2013,57(4):1723-1729.
doi: 10.1128/AAC.02306-12 pmid: 23357760 |
| [39] |
Takahashi N, Tanabe K, Tsukahara T, et al. Large-scale survey for novel genotypes of Plasmodium falciparum chloroquine-resistance gene Pfcrt[J]. Malar J, 2012,11:92.
doi: 10.1186/1475-2875-11-92 pmid: 22453078 |
| [40] |
Mbaisi A, Liyala P, Eyase F, et al. Drug susceptibility and genetic evaluation of Plasmodium falciparum isolates obtained in four distinct geographical regions of Kenya[J]. Antimicrob Agents Chemother, 2004,48(9):3598-3601.
doi: 10.1128/AAC.48.9.3598-3601.2004 pmid: 15328137 |
| [41] | Song J. Study of chloroquine-resistant gene Pfmdr1 polymorphisms of Plasmodium falciparum from Hainan Province[J]. J Trop Med, 2005,5(1):26-29. (in Chinese) |
| ( 宋杰. 海南省恶性疟原虫氯喹抗性相关基因 Pfmdr1多态性分析[J]. 热带医学杂志, 2005,5(1):26-29.) | |
| [42] | Guan YY, Zhang GQ, Hu L, et al. Relationships between chloroquine resistance and polymorphisms in Pfcrt and Pfmdr1 in Plasmodium falciparum isolated from China[J]. J Pathog Biol, 2009,4(1):27-31. (in Chinese) |
| ( 官亚宜, 张国庆, 胡铃, 等. 我国恶性疟原虫Pfcrt和Pfmdr1基因多态性及与氯喹敏感性关系的研究[J]. 中国病原生物学杂志, 2009,4(1):27-31.) | |
| [43] |
Feng J, Zhou D, Lin Y, et al. Amplification of Pfmdr1, Pfcrt, Pvmdr1, and K13 propeller polymorphisms associated with Plasmodium falciparum and Plasmodium vivax isolates from the China-Myanmar border[J]. Antimicrob Agents Chemother, 2015,59(5):2554-2559.
doi: 10.1128/AAC.04843-14 pmid: 25691632 |
| [44] |
Zhang J, Li N, Siddiqui FA, et al. In vitro susceptibility of Plasmodium falciparum isolates from the China-Myanmar border area to artemisinins and correlation with K13 mutations[J]. Int J Parasitol Drugs Drug Resist, 2019,10:20-27.
doi: 10.1016/j.ijpddr.2019.04.002 pmid: 31009824 |
| [45] | Gao Q, Tang LH, Fu LC, et al. WS/T 485-2016 The guidelines for the use of antimalarial drugs[S]. Beijing: National Health and Family Planning Commission, PRC, 2016. (in Chinese) |
| ( 高琪, 汤林华, 符林春, 等. WS/T 485-2016 抗疟药使用规范[S]. 北京: 中华人民共和国国家卫生和计划生育委员会, 2016. |
| [1] | ZHOU Ruimin, JI Penghui, LI Suhua, YANG Chengyun, LIU Ying, QIAN Dan, DENG Yan, LU Deling, ZHAO Yuling, ZHAO Dongyang, ZHANG Hongwei. Polymorphism analysis of drug resistance genes in imported Plasmodium falciparum isolates from Equatorial Guinea in Henan Province [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(5): 593-600. |
| [2] | XU Shaojie, CHEN Shenbo, CHEN Junhu. Research progress on transcription regulation of rif gene in Plasmodium falciparum [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(3): 374-379. |
| [3] | TIAN Bin, LIAO Yu, WEN Lan, XIAO Fang, ZHANG Bin, SHEN Xiao-jun. Analysis on the copy number variation of multidrug resistance-1 gene in 122 imported cases of falciparum malaria in Changsha [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(1): 127-131. |
| [4] | SHI Ming-li, XIAO Bo, JIANG Lu-bin. Research progress on the expression regulation of var genes in Plasmodium falciparum [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(6): 719-724. |
| [5] | LI Mei, TU Hong, XIA Zhi-gui, WANG Zhen-yu, ZHOU He-jun. Thermal stability of diagnostic targets Plasmodium falciparum histidine rich protein Ⅱ and Plasmodium lactate dehydrogenase in rapid detection [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(2): 245-248. |
| [6] | SHI Shan-mei, CHEN Jun-hu. Research progress on the structure and function of RIFIN protein of Plasmodium falciparum [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(2): 249-255. |
| [7] | YE Sheng-yu, CHENG Yi-yi, LI Man, ZHOU Hong-ning. An overview on the resistance of Plasmodium falciparum to primary anti-malarial drugs in China [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2020, 38(5): 631-636. |
| [8] | YE Sheng-yu, CHENG Yi-yi, LI Man, ZHOU Hong-ning. Advances in methods for detecting drug-resistance molecular markers of Plasmodium falciparum [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2020, 38(4): 490-495. |
| [9] | Yun-shan MOU, Lu-jie LI, Yin-juan WU, Xue-rong LI. Exploration of molecular mechanisms of artemisinin resistance in malaria parasites [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2018, 36(6): 636-642. |
| [10] | Chun LIU, ALFRED Ndoumadiamba, Gou GNONDA Mounzie. Clinical application of colloidal-gold detection reagent for Plasmodium falciparum in Gabon of Africa [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2018, 36(6): 679-680. |
| [11] | Qiang MAO, Fu-quan PEI, Yong-zhen CEN, Meng-ran LIU, Hao ZHANG, Zhuo-hui DENG. Laboratory testing and traceability analysis of a case of transfusion-transmitted falciparum malaria in Guangdong Province [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2018, 36(5): 529-533. |
| [12] | Zhi-hua WANG, Chun-yan WEI, Heng WANG. Research development on non-coding RNA of Plasmodium falciparum [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2018, 36(4): 409-413. |
| [13] | Cheng-yun YANG, Su-hua LI, Ya-lan ZHANG, Rui-min ZHOU, Ying LIU, Dan QIAN, Yu-ling ZHAO, Bian-li XU, Hong-wei ZHANG, Yan DENG. Analysis of mutations of Plasmodium falciparum multidrug resistance gene 1 and K13 gene in imported Plasmodium falciparum in Henan Province [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2018, 36(2): 97-102. |
| [14] | Xi-shuai JIA, Shui-mao ZHOU, Yan YANG, Ming-xing XU, Kai WU. Genotyping of merozoite surface protein 1 and 2 of imported Plasmodium falciparum in Wuhan City [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2017, 35(5): 434-439. |
| [15] | Yi-ni TIAN, Run YE, Wei-qing PAN, Dong-mei ZHANG. Approaches to screening and identifying genes associated with drug-resistance of Plasmodium falciparum [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2017, 35(5): 495-498. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||