
CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES ›› 2019, Vol. 37 ›› Issue (2): 161-167.doi: 10.12140/j.issn.1000-7423.2019.02.008
• ORGINAL ARTICLES • Previous Articles Next Articles
Jing LIU1( ), Jyh-wei SHIN2, Pei PEI1, Xiao-yin FU1, De-jun LIAO3, Yun-yu LU1, Sa XUE1, Chen-chieh LIAO4, Deng-yu LIU1,*(
), Jyh-wei SHIN2, Pei PEI1, Xiao-yin FU1, De-jun LIAO3, Yun-yu LU1, Sa XUE1, Chen-chieh LIAO4, Deng-yu LIU1,*( )
)
Received:2018-11-29
Online:2019-04-30
Published:2019-05-13
Contact:
Deng-yu LIU
E-mail:1061102060@qq.com;liudengyu@tom.com
Supported by:CLC Number:
Jing LIU, Jyh-wei SHIN, Pei PEI, Xiao-yin FU, De-jun LIAO, Yun-yu LU, Sa XUE, Chen-chieh LIAO, Deng-yu LIU. A modified method to infect Blastocystis hominis in rats and the pathological changes after infection[J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2019, 37(2): 161-167.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.jsczz.cn/EN/10.12140/j.issn.1000-7423.2019.02.008
Table 1
Rats infected with B. hominis determined by in vitro culture
| 组别 Group | 鼠数 No. | 粪便培养阳性鼠数 No. positives in fecal culture | 肠内容物培养阳性鼠数 No. positives in intestinal content culture | ||||
|---|---|---|---|---|---|---|---|
| 3 d | 6 d | 9 d | 12 d | 18 d | 21 d | ||
| 对照组Control group | 6 | 0 | 0 | 0 | 0 | 0 | 0 |
| 105感染组105 infection group | 6 | 0 | 2 | 2 | 2 | 2 | 2 |
| 106感染组106 infection group | 6 | 4 | 4 | 3 | 1 | 2 | 3 |
| 107感染组107 infection group | 6 | 5 | 6 | 5 | 5 | 5 | 5 |
| 108感染组108 infection group | 6 | 5 | 6 | 6 | 6 | 6 | 6 |
Fig. 2
Morphological comparison between B. hominis isolated from infected rats and the original strain for infection(× 400)A, B, C: Direct smear of B. hominis; D, E, F: Iodine staining of B. hominis. A, D: original strain of B. hominis for infection; B, E: The 72 h cultured B. hominis in fecal samples of the 21st day after infection; C, F: The 144 h cultured B. hominis for 144 hours in the fecal samples of the 21st day after infection. Arrows indicate the trophozoites
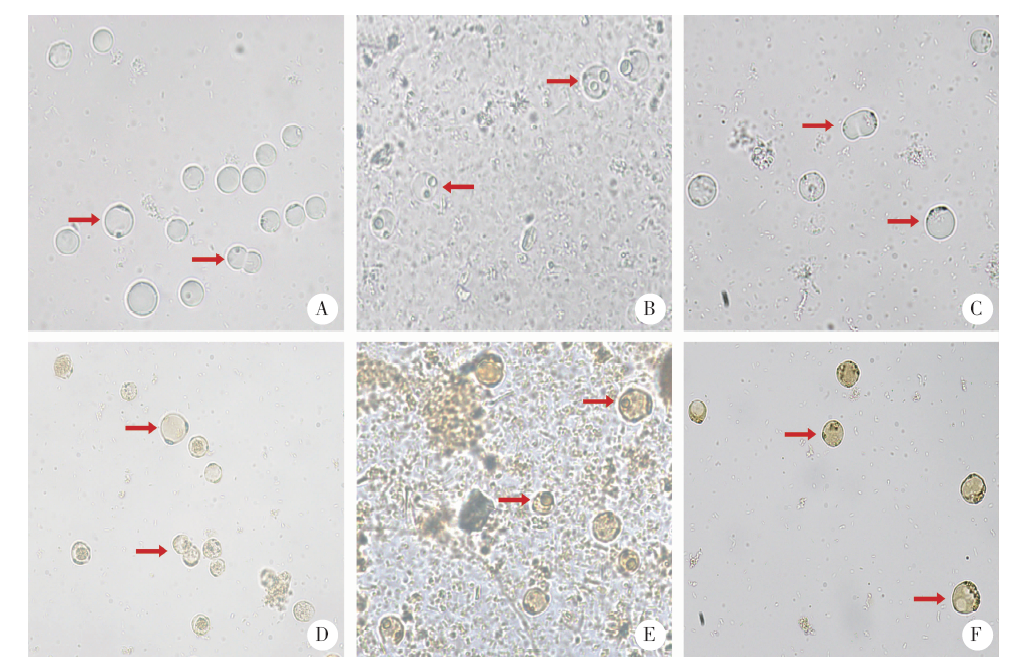
Table 2
Infection intensity of B. hominis in the contents of various intestinal segments of infected rats
| 组别 Group | 人芽囊原虫密度/104·ml-1 B. hominis intensity /104·ml-1 | ||||
|---|---|---|---|---|---|
| 十二指肠Duodenum | 空肠Jejunum | 回肠Ileum | 盲肠Caecum | 结肠Colon | |
| 对照组Control group | 0 | 0 | 0 | 0 | 0 |
| 105感染组105 infection group | 0 | 0 | 0 | 4.3 ± 1.1 | 55.8 ± 74.2 |
| 106感染组106 infection group | 0 | 0 | 3.0 ± 4.2 | 84.7 ± 141.1 | 167.2 ± 151.9 |
| 107感染组107 infection group | 0 | 0 | 13.6 ± 20.0 | 284.9 ± 254.6 | 187.5 ± 157.1 |
| 108感染组108 infection group | 0 | 0 | 0.2 ± 0.3 | 45.9 ± 60.6 | 105.0 ± 130.4 |
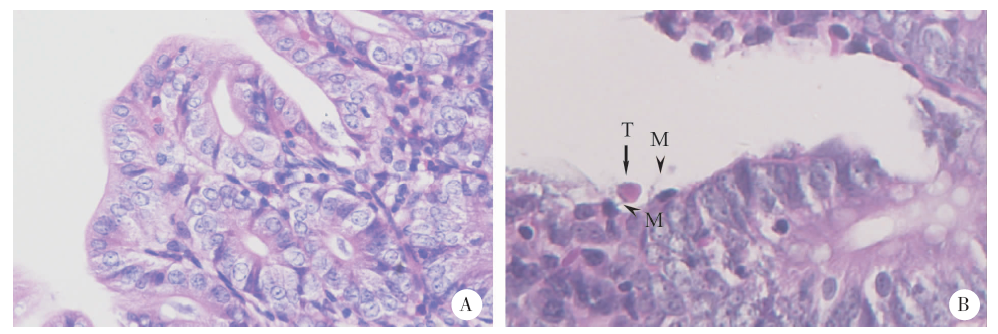
Fig. 3
Sections of cecum of rats infected with 108 B. hominis for 21 days (HE staining, × 400)A: Control group, the tissue structure was intact, the mucosal villi were arranged regularly, and the cell morphology was normal; B: 108 infected rat cecum with trophozoites identified (T), and mucin (M) bound to it
| [1] | Tan KS.Blastocystis in humans and animals: new insights using modern methodologies[J]. Vet Parasitol, 2004, 126(1/2): 121-144. |
| [2] | Duda A, Kosikbogacka D, Lanochaarendarczyk N, et al. The prevalence of Blastocystis hominis and other protozoan parasites in soldiers returning from peacekeeping missions[J]. Am J Trop Med Hyg, 2015, 92(4): 805-806. |
| [3] | Ocana-Losada C, Cuenca-Gomez JA, Cabezas-Fernandez MT, et al. Clinical and epidemiological characteristics of intestinal parasite infection by Blastocystis hominis[J]. Rev Clin Esp, 2018, 218(3): 115-120. |
| [4] | 辛致炜, 廖振捷, 廖德君, 等. 人芽囊原虫体外纯培养法的改良及形态观察[J]. 中国寄生虫学与寄生虫病杂志, 2017, 35(6): 623-625. |
| [5] | Zhou XB, Zhang X, Qiao JY, et al. Encystation: survival of Blastocystis hominis in immunocompetent mice abdomen cavity[J]. Parasitol Res, 2010, 106(6): 1315-1320. |
| [6] | Moe KT, Singh M, Gopalakrishnakone P, et al. Cytopathic effect of Blastocystis hominis after intramuscular inoculation into laboratory mice[J]. Parasitol Res, 1998, 84(6): 450-454. |
| [7] | 姚繁荣, 乔继英, 赵晏, 等. 人芽囊原虫感染小鼠试验[J]. 中国寄生虫学与寄生虫病杂志, 2005, 23(6): 444-448. |
| [8] | Ajjampur SS, Png CW, Chia WN, et al. Ex vivo and in vivo mice models to study Blastocystis spp. adhesion, colonization and pathology: closer to proving koch’s postulates[J]. PLoS One, 2016, 11(8): e0160458. |
| [9] | Yoshikawa H, Yoshida K, Nakajima A, et al. Fecal-oral transmission of the cyst form of Blastocystis hominis in rats[J]. Parasitol Res, 2004, 94(6): 391-396. |
| [10] | 李娟, 邓婷, 李小花, 等. 人芽囊原虫感染大鼠模型的建立[J]. 赣南医学院学报, 2013, 33(1): 4-7. |
| [11] | 王东, 薛长贵. 人芽囊原虫不同感染途径对免疫抑制小鼠肠黏膜的影响[J]. 现代预防医学, 2012, 39(18): 4813-4815. |
| [12] | Elwakil HS, Hewedi IH.Pathogenic potential of Blastocystis hominis in laboratory mice[J]. Parasitol Res, 2010, 107(3): 685-689. |
| [13] | Moe KT, Singh M, Howe J, et al. Experimental Blastocystis hominis infection in laboratory mice[J]. Parasitol Res, 1997, 83(4): 319-325. |
| [14] | 张红卫, 李文, 颜秋叶, 等. 人芽囊原虫对实验感染昆明小鼠肠黏膜超微结构的影响[J]. 中国寄生虫学与寄生虫病杂志, 2006, 24(3): 187-191. |
| [15] | Stensvold CR, Suresh GK, Tan KS, et al. Terminology for Blastocystis subtypes: a consensus[J]. Trends Parasitol, 2007, 23(3): 93-96. |
| [16] | Yason JA, Ajjampur SSR, Tan KSW.Blastocystis isolate B exhibits multiple modes of resistance against antimicrobial peptide LL-37[J]. Infect Immun, 2016, 84(8): 2220-2232. |
| [17] | 孙煦苧, 廖德君, 刘静, 等. mLES法培养人芽囊原虫的实验观察[J]. 中国病原生物学杂志, 2018, 13(3): 259-262, 266. |
| [18] | Mohamed RT, El-Bali MA, Mohamed AA, et al. Subtyping of Blastocystis sp. isolated from symptomatic and asymptomatic individuals in Makkah, Saudi Arabia[J]. Parasit Vectors, 2017, 10(1): 174. |
| [19] | Windsor JJ, Macfarlane L, Hughes-Thapa G, et al. Incidence of Blastocystis hominis in faecal samples submitted for routine microbiological analysis[J]. Br J Biomed Sci, 2002, 59(3): 154-157. |
| [20] | Hirata T, Nakamura H, Kinjo N, et al. Prevalence of Blastocystis hominis and Strongyloides stercoralis infection in Okinawa, Japan[J]. Parasitol Res, 2007, 101(6): 1717-1719. |
| [21] | Wong KH, Ng GC, Lin RT, et al. Predominance of subtype 3 among Blastocystis isolates from a major hospital in Singapore[J]. Parasitol Res, 2008, 102(4): 663-670. |
| [22] | Zhang WZ, Ren GX, Zhao W, et al. Genotyping of enterocytozoon bieneusi and subtyping of Blastocystis in cancer patients: relationship to diarrhea and assessment of zoonotic transmission[J]. Front Microbiol, 2017, 8: 1835. |
| [23] | Zhang SX, Yang CL, Gu WP, et al. Case-control study of diarrheal disease etiology in individuals over 5 years in southwest China[J]. Gut Pathog, 2016, 8: 58. |
| [24] | Tian LG, Wang TP, Lv S, et al. HIV and intestinal parasite co-infections among a Chinese population: an immunological profile[J]. Infect Dis Poverty, 2013, 2: 18. |
| [25] | 胡缨, 李艳文, 刘晓泉, 等. 慢性病患者合并人芽囊原虫感染的情况调查[J]. 中国卫生检验杂志, 2017, 27(17): 2558-2560. |
| [26] | Liu DY, Lu ZC, Liu XQ, et al. Comparative analysis of intestinal parasitic infections of outpatients in Guangxi medical university affiliated hospital in 2005 and 2013[J]. Int J Clin Exp Med, 2015, 8(9): 16640-16645. |
| [27] | 何姗姗, 伍玲园, 刘晓泉, 等. 巴马瑶族自治县人芽囊原虫感染情况调查[J]. 中国寄生虫学与寄生虫病杂志, 2013, 31(1): 76-77. |
| [28] | Defaye M, Nourrisson C, Baudu E, et al. Efficient and reproducible experimental infections of rats with Blastocystis spp.[J]. PLoS One, 2018, 13(11): e0207669. |
| [29] | Chandramathi S, Suresh KG, Mahmood AA, et al. Urinary hyaluronidase activity in rats infected with Blastocystis hominis: evidence for invasion?[J]. Parasitol Res, 2010, 106(6): 1459-1463. |
| [30] | Chandramathi S, Suresh K, Sivanandam S, et al. Stress exacerbates infectivity and pathogenicity of Blastocystis hominis: in vitro and in vivo evidences[J]. PLoS One, 2014, 9(5): e94567. |
| [31] | Suresh K, Venilla GD, Tan TC, et al. In vivo encystation of Blastocystis hominis[J]. Parasitol Res, 2009, 104(6): 1373-1380. |
| [32] | Ragavan ND, Govind SK, Chye TT, et al. Factors that influence the shedding of Blastocystis cysts in an irritable bowel syndrome (IBS) patient: an evidence-based case study[J]. Parasitol Res, 2015, 114(8): 2999-3005. |
| [33] | 苗雅娟, 吴秀萍, 王光明, 等. 黏蛋白在肠道寄生虫感染中的作用[J]. 湖北农业科学, 2012, 51(12): 2409-2411, 2415. |
| [1] | XUE Yushan, LIN Ping, CHENG Xunjia, FENG Meng. Damage caused by chronic infection of Toxoplasma gondii on the host central nervous system and its mechanism [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(5): 527-531. |
| [2] | GUO Shuai, HE Biao, GAO Yuanli, FAN Yongling, ZHU Feng, DING Yan, LIU Taiping, XU Wenyue. Specie-specific analysis of plasmodia infecting rats and mice [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(5): 539-545. |
| [3] | LIU Wenhu, HUANG Ming, LIANG Jin, LIU Jianxiong, WEN Zhaomeng, MA Shaobo. A case of ventricular cysticercosis complicated with hydrocephalus [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(5): 644-646. |
| [4] | ZHANG Li, MIAO Feng, SHEN Yanmei. A case of Capillaria hepatica infection [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(5): 650-652. |
| [5] | LI Xiaoli, LI Shaogang, WU Zhaoyong. Clinical characteristics of patients with intestinal Diphyllobothrium tapeworm infection [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(4): 459-463. |
| [6] | ZHENG Yuhua, TIE Ping, BAI Yongfei, YAN Changfu, WANG Ting, WANG Jingying, TIAN Xiaodong, DAI Peifang. Investigation on visceral leishmaniasis in domestic dogs and sandfly density in epidemic area in Shanxi Province from 2021 to 2022 [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(4): 470-475. |
| [7] | LI Yuqiong, YU Youli, GAO Junrong, LIU Yunyun, LI Hongbing, NIU Xiaohao. Enterocytozoon bieneusi infection in dairy cows and its genotype identification in Yinchuan area of Ningxia Province [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(4): 476-479. |
| [8] | WANG Feng, WU Fan, LI Linlin, HUANG Qingqing. Prevalence of parasitic infections in wild mice in Wuhu City, Anhui Province [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(4): 516-519. |
| [9] | ZHU Canmin, PENG Weijian, WANG Dili, ZHOU Huajing, JIN Qiangjian, CHANG Chang. A case of acute primary amoebic meningoencephalitis [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(4): 524-526. |
| [10] | ZHANG Mizhen, HUANG Jilei, ZHU Huihui, ZHOU Changhai, ZHU Tingjun, QIAN Menbao, CHEN Yingdan, LI Shizhu. Epidemiological analysis of soil-transmitted nematode infections in China in 2020 [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(3): 331-335. |
| [11] | GUO Gang, REN Yuan, JIAO Hongjie, WU Juan, GUO Baoping, QI Wenjing, LI Jun, ZHANG Wenbao. Effect of intraperitoneal inoculation with Echinococcus microcysts on the infection and pathogenicity of E. multilocularis in mouse liver [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(2): 156-162. |
| [12] | LI Chang, DU Xinyue, YAN Min, WANG Zhaojun. Research advances on the role and mechanism of neutrophil extracellular traps in parasitic infection [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(2): 219-222. |
| [13] | ZHENG Dan, LIN Chenxin, CAI Wuwei, XIE Hangguo. Investigation of Anisakis spp. infection in marine fish in Fujian Province, 2019—2021 [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(2): 238-240. |
| [14] | ZHANG Fengyu, LIU Liu, ZHANG Jing, ZHANG Hao. Prevalence of trematode metacercariae in Pseudorasbora parva and species identification in Qiqihaer area [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(1): 112-116. |
| [15] | ZHU Mingchao, ZHU Ya, ZHAO Jianzhong, YUAN Huizhen. Analysis of Blastocystis hominis infection in long-term hospitalized patients in Tianmen City [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(1): 121-124. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||