
CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES ›› 2023, Vol. 41 ›› Issue (4): 434-439.doi: 10.12140/j.issn.1000-7423.2023.04.006
• ORIGINAL ARTICLES • Previous Articles Next Articles
FENG Xiaoxiao1,2( ), BAIMA Yangjin1, ZHANG Ting3, LU Haojie2, WEI Liming2,*(
), BAIMA Yangjin1, ZHANG Ting3, LU Haojie2, WEI Liming2,*( )
)
Received:2023-02-20
Revised:2023-06-17
Online:2023-08-30
Published:2023-09-06
Contact:
*E-mail: Supported by:CLC Number:
FENG Xiaoxiao, BAIMA Yangjin, ZHANG Ting, LU Haojie, WEI Liming. Applied research of serum IgG sialylation modification in the diagnosis of hepatic echinococcosis[J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(4): 434-439.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.jsczz.cn/EN/10.12140/j.issn.1000-7423.2023.04.006
Table 1
Amount of serum IgG sialylation modification in each group
| 分组 group | 单唾液酸 化含量/% Mono-sialization content/% | 双唾液酸 化含量/% Di-sialization content/% | 总唾液酸 化含量/% Total sialylation content/% | P1值 P1 value |
|---|---|---|---|---|
| 健康对照 Healthy control group | 16.55 ± 2.95 | 3.71 ± 1.04 | 20.26 ± 3.79 | > 0.10 |
| 肝细粒棘球蚴病组 Hepatic cystic echinococcosis group | 14.15 ± 2.89 | 3.02 ± 0.98 | 17.25 ± 3.69 | > 0.10 |
| 肝多房棘球蚴病组 Hepatic alveolar echinococcosis group | 10.24 ± 2.97 | 1.92 ± 0.65 | 12.27 ± 3.65 | > 0.10 |
| 肝癌组 Hepatocellular carcinoma group | 15.09 ± 2.68 | 3.28 ± 1.30 | 18.37 ± 3.69 | > 0.10 |
| P1值 P1 value | > 0.10 | > 0.10 | > 0.10 | |
| P2值 P2 value | < 0.000 1 | < 0.000 1 | < 0.000 1 |

Fig. 2
Scatter plot of the amount of different types of IgG sialylation modification in each group A: Mono-sialylation modification; B: Bi-sialylation modification; C: Total sialylation modification. N: Healthy control group; CE: Hepatic cystic echinococcosis group; AE: Hepatic alveolar echinococcosis group; HCC: Hepatocellular carcinoma group. a, P < 0.001; b, P < 0.000 1.
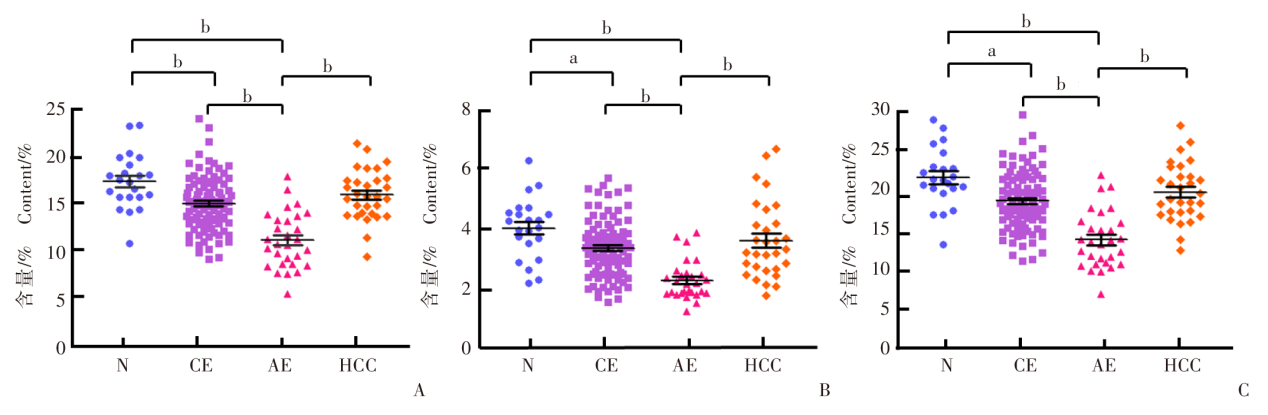

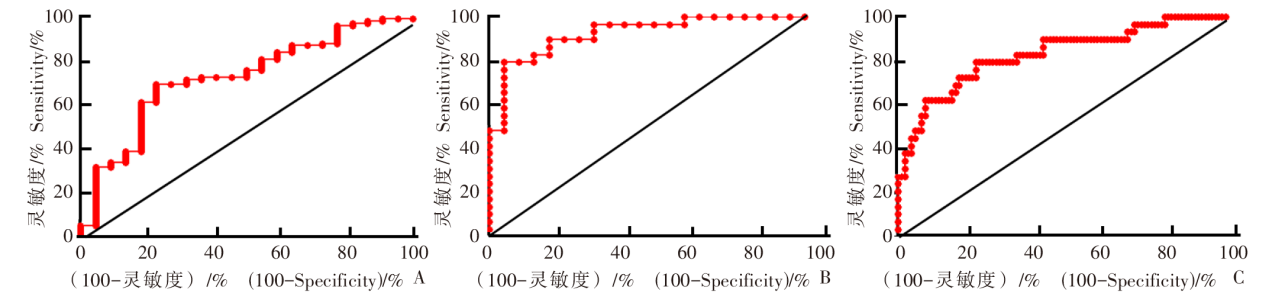
Fig. 3
Receiver operating characteristic curve for identification of IgG sialylation modification in each group A: Comparison of hepatic cystic echinococcosis group and healthy control group; B: Comparison of hepatic alveolar echinococcosis group and healthy control group; C: Comparison of hepatic cystic echinococcosis group and hepatic alveolar echinococcosis group.
| [1] |
Acosta-Jamett G, Hernández FA, Castro N, et al. Prevalence rate and risk factors of human cystic echinococcosis: a cross-sectional, community-based, abdominal ultrasound study in rural and urban north-central Chile[J]. PLoS Negl Trop Dis, 2022, 16(3): e0010280.
doi: 10.1371/journal.pntd.0010280 |
| [2] |
Wang LY, Qin M, Liu ZH, et al. Prevalence and spatial distribution characteristics of human echinococcosis in China[J]. PLoS Negl Trop Dis, 2021, 15(12): e0009996.
doi: 10.1371/journal.pntd.0009996 |
| [3] | Xu K, Wang ZX, Fan HN, et al. Hepatic echinococcosis misdiagnosed as liver cancer: a case report[J]. Chin J ParasitolParasit Dis, 2021, 39(3) :717-719. (in Chinese) |
| (徐凯, 王志鑫, 樊海宁, 等. 肝棘球蚴病误诊肝癌1例报告[J]. 中国寄生虫学与寄生虫病杂志, 2021, 39(3): 717-719.) | |
| [4] |
Kalifu B, Meng Y, Maimaitinijiati Y, et al. Radical resection of hepatic polycystic echinococcosis complicated with hepatocellular carcinoma: a case report[J]. World J Clin Cases, 2021, 9(3): 659-665.
doi: 10.12998/wjcc.v9.i3.659 pmid: 33553405 |
| [5] |
(Kazuaki, Ohtsubo. Glycosylation in cellular mechanisms of health and disease[J]. Cell, 2006, 126(5): 855-867.
doi: 10.1016/j.cell.2006.08.019 pmid: 16959566 |
| [6] |
Rudd PM, Elliott T, Cresswell P, et al. Glycosylation and the immune system[J]. Science, 2001, 291(5512): 2370-2376.
doi: 10.1126/science.291.5512.2370 pmid: 11269318 |
| [7] |
Bagdonaite I, Wandall HH. Global aspects of viral glycosylation[J]. Glycobiology, 2018, 28(7): 443-467.
doi: 10.1093/glycob/cwy021 pmid: 29579213 |
| [8] |
Narimatsu Y, Büll C, Chen YH, et al. Genetic glycoengineering in mammalian cells[J]. J Biol Chem, 2021, 296: 100448.
doi: 10.1016/j.jbc.2021.100448 |
| [9] |
Liu D, Li QH, Zhang XY, et al. Systematic review: immunoglobulin G N-glycans as next-generation diagnostic biomarkers for common chronic diseases[J]. OMICS, 2019, 23(12): 607-614.
doi: 10.1089/omi.2019.0032 pmid: 31414971 |
| [10] |
Pincetic A, Bournazos S, Dilillo DJ, et al. Type I and type II Fc receptors regulate innate and adaptive immunity[J]. Nat Immunol, 2014, 15(8): 707-716.
doi: 10.1038/ni.2939 pmid: 25045879 |
| [11] |
Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system?[J]. Nat Rev Immunol, 2013, 13(3): 176-189.
doi: 10.1038/nri3401 pmid: 23411799 |
| [12] |
Thomas D, Rathinavel AK, Radhakrishnan P. Altered glycosylation in cancer: a promising target for biomarkers and therapeutics[J]. Biochim Biophys Acta Rev Cancer, 2021, 1875(1): 188464.
doi: 10.1016/j.bbcan.2020.188464 |
| [13] |
Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications[J]. Nat Rev Cancer, 2015, 15(9): 540-555.
doi: 10.1038/nrc3982 pmid: 26289314 |
| [14] |
Plomp R, Bondt A, de Haan N, et al. Recent advances in clinical glycoproteomics of immunoglobulins[J]. Mol Cell Proteomics, 2016, 15(7): 2217-2228.
doi: 10.1074/mcp.O116.058503 |
| [15] |
Qin WJ, Pei H, Qin RH, et al. Alteration of serum IgG galactosylation as a potential biomarker for diagnosis of neuroblastoma[J]. J Cancer, 2018, 9(5): 906-913.
doi: 10.7150/jca.22014 pmid: 29581769 |
| [16] |
Harvey DJ. Electrospray mass spectrometry and fragmentation of N-linked carbohydrates derivatized at the reducing terminus[J]. J Am Soc Mass Spectrom, 2000, 11(10): 900-915.
doi: 10.1016/S1044-0305(00)00156-2 |
| [17] |
Keser T, Pavić T, Lauc G, et al. Comparison of 2-aminobenzamide, procainamide and RapiFluor-MS as derivatizing agents for high-throughput HILIC-UPLC-FLR-MS N-glycan analysis[J]. Front Chem, 2018, 6: 324.
doi: 10.3389/fchem.2018.00324 pmid: 30094234 |
| [18] |
Varki NM, Varki A. Diversity in cell surface sialic acid presentations: implications for biology and disease[J]. Lab Invest, 2007, 87(9): 851-857.
doi: 10.1038/labinvest.3700656 pmid: 17632542 |
| [19] |
Zhang Y, Wang RH, Feng Y, et al. The role of sialyltransferases in gynecological malignant tumors[J]. Life Sci, 2020, 263: 118670.
doi: 10.1016/j.lfs.2020.118670 |
| [20] |
Bhide GP, Colley KJ. Sialylation of N-glycans: mechanism, cellular compartmentalization and function[J]. Histochem Cell Biol, 2017, 147(2): 149-174.
doi: 10.1007/s00418-016-1520-x pmid: 27975143 |
| [21] |
Pietrobono S, Stecca B. Aberrant sialylation in cancer: biomarker and potential target for therapeutic intervention?[J]. Cancers, 2021, 13(9): 2014.
doi: 10.3390/cancers13092014 |
| [22] | Ou LL, He XZ, Liu NH, et al. Sialylation of FGFR1 by ST6Gal‑Ⅰ overexpression contributes to ovarian cancer cell migration and chemoresistance[J]. Mol Med Rep, 2020, 21(3): 1449-1460. |
| [23] |
Wang ZH, Geng ZH, Shao WW, et al. Cancer-derived sialylated IgG promotes tumor immune escape by binding to siglecs on effector T cells[J]. Cell Mol Immunol, 2020, 17(11): 1148-1162.
doi: 10.1038/s41423-019-0327-9 |
| [24] |
Gudelj I, Lauc G, Pezer M. Immunoglobulin G glycosylation in aging and diseases[J]. Cell Immunol, 2018, 333: 65-79.
doi: S0008-8749(18)30325-3 pmid: 30107893 |
| [25] |
Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation[J]. Science, 2006, 313(5787): 670-673.
doi: 10.1126/science.1129594 pmid: 16888140 |
| [26] |
Quast I, Keller CW, Maurer MA, et al. Sialylation of IgG Fc domain impairs complement-dependent cytotoxicity[J]. J Clin Invest, 2015, 125(11): 4160-4170.
doi: 10.1172/JCI82695 pmid: 26436649 |
| [1] | LI Benfu, YANG Jing, YANG Jinyu, LUO Ruijuan, ZHU Binlin, CHEN Tailin, ZHANG Lijuan, LI Xueyao, YAN Xinliu, ZI Jinrong, PENG Jia, WANG Zhengqing, LI Jianxiong, CAI Xuan, XU Qian, WU Fangwei, YANG Yaming. Epidemiological investigation and case retrospective analysis of echinococcosis in Dali Prefecture, Yunnan Province [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(5): 573-578. |
| [2] | NI Bixian, XU Xiangzhen, ZHANG Qiang, TANG Feng, ZHANG Jiayao, MAO Fanzhen, DAI Yang, LIU Yaobao, CAO Jun. Epidemiological characteristics of echinococcosis cases reported in the National Notifiable Disease Report System in Jiangsu Province, 2015—2022 [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(5): 636-639. |
| [3] | ZHU Aiya, WANG Xu, WANG Jiangyou, WANG Ying, LI Yang, SONG Shan, GENG Yan, LAN Ziyao, DAI Jiarui. A child case of alveolar echinococcosis in Guizhou Province [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(4): 520-523. |
| [4] | RAOWAN Tuolehong, ABUDUSALAMU Abulikemu, YANG Lingfei, CHEN Lu, LI Zhao, JIA Fang, SONG Tao. Effect evaluation and factor analysis of ultrasonic manifestations in the diagnosis of hepatic alveolar echinococcosis [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(3): 312-318. |
| [5] | WANG Wei, CAI Huixia. Surveillance of echinococcosis in Qinghai Province in 2020 [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(3): 319-324. |
| [6] | KUI Yan, XUE Chuizhao, WANG Xu, LIU Baixue, WANG Ying, WANG Liying, YANG Shijie, HAN Shuai, WU Weiping, XIAO Ning. Progress of echinococcosis control in China, 2021 [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(2): 142-148. |
| [7] | MA Bingcun, LIU Yuying, ZHANG Tiantian, LEI Wen, MA Xiao, LIU Shou. A case-control study on the influencing factors of echinococcosis in Qinghai Province [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(2): 183-191. |
| [8] | MA Hui, CHONG Shigui, CHEN Gen, ZHANG Linghui, QIN Junmei, ZHAO Yumin. Research progress on the cellular signal pathways associated in alveolar echinococcosis [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(2): 223-227. |
| [9] | LU Weimin, YANG Xiaotao, ZHU Ying, ZHANG Hong, LI Jiwei, WANG Yanchun. A child case of pulmonary cystic echinococcosis [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(2): 253-256. |
| [10] | CAO Deping, WU Defang, PANG Mingquan, PENG Xiaohong, LI Dayu, FAN Haining. Difference analysis of the gut microbiome in patients with echinococcosis [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(1): 103-107. |
| [11] | DUAN Hongju, LI Lingjie, WU Xianglin, YAN Fang, QI Rongting, MA Tianbo. Analysis of hospitalization expenses and influencing factors in surgery for patients with cystic echinococcosis in Ningxia during 2016—2021 [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(1): 75-80. |
| [12] | WU Xiang-lin, YAN Fang, DUAN Hong-ju, QI Rong-ting, MA Tian-bo, GAO Jian-wei. Echinococcus multilocularis infection in dogs and other wild hosts in endemic area of Ningxia in 2021 [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(6): 717-722. |
| [13] | LIU Yu-ying, ZHANG Tian-tian, MA Xiao, LEI Wen, MA Bing-cun, LIU Shou. Awareness and influencing factors of knowledge on echinococcosis prevention and control among adults in Qinghai Province [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(6): 723-729. |
| [14] | AN Xiu-qing, WANG Miao-miao, ZHOU Hong-qian, MENG Kai, CAI Jian-ping, LIU Guang-hui, A Ji-de, YANG Jing-yu. Research progress on microvascular density in hepatic alveolar echinococcosis [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(6): 792-797. |
| [15] | GUO Lu, WU Xiao-xia, DUAN Lan-li, WANG Bing-jie, XU Ning, AREAI Ahatai, WU Yun-hua, ZHAO Li, BAN Wan-li, CHEN Yun-ying, YU Wan-rong, LIU Shuai, PAN Xing-yu, WULIJIANG Kamali, XU Jing, MUNILA Teliewuhan, ZHANG Zhuang-zhi. Prevalence and gene polymorphism analysis of Echinococcus granulosus in cattle and sheep in part areas of Xinjiang [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(5): 603-609. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||