
CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES ›› 2023, Vol. 41 ›› Issue (1): 29-35.doi: 10.12140/j.issn.1000-7423.2023.01.005
• ORIGINAL ARTICLES • Previous Articles Next Articles
SUN Jiahui( ), SONG Peng, CHEN Muxin, ZHOU Yan, LIN Lin, CHEN Jiaxu, CAI Yuchun*(
), SONG Peng, CHEN Muxin, ZHOU Yan, LIN Lin, CHEN Jiaxu, CAI Yuchun*( )
)
Received:2022-10-17
Revised:2022-11-10
Online:2023-02-28
Published:2022-12-27
Contact:
* E-mail: Supported by:CLC Number:
SUN Jiahui, SONG Peng, CHEN Muxin, ZHOU Yan, LIN Lin, CHEN Jiaxu, CAI Yuchun. Expression and functional analysis of recombinant peptidyl-prolyl cis-trans isomerase gene of Babesia microti[J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(1): 29-35.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.jsczz.cn/EN/10.12140/j.issn.1000-7423.2023.01.005
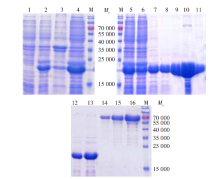
Fig. 2
Prokaryotic expression and purification of BmPPIase M: Protein marker; 1: Uninducted total cell lysate; 2: Induced total bacterial lysate of BmPPIase; 3: Precipitation of induced protein; 4: Supernatant of induced protein; 5: Pre-purified protein; 6: Flow in liquid of protein; 7-11: Purified protein in different concentration (10, 20, 50, 200, 500 mmol/L midazole eluent); 12-13: Recombinant BmPPIase protein (1, 2 μl); 14-16: BSA standard protein (1, 2, 4 μg).
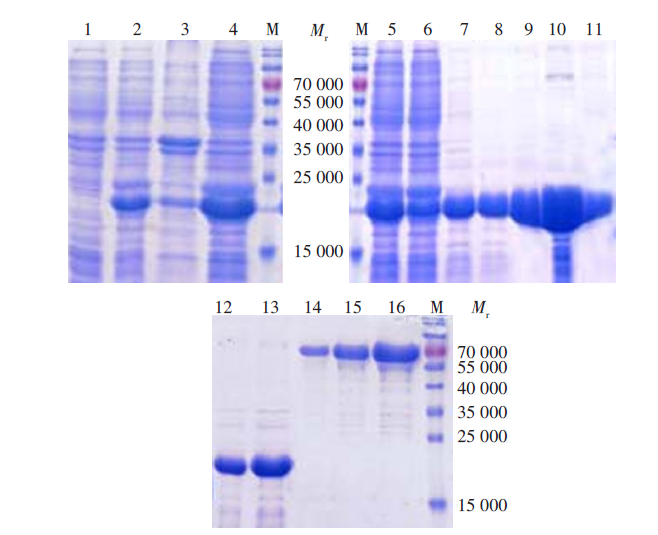
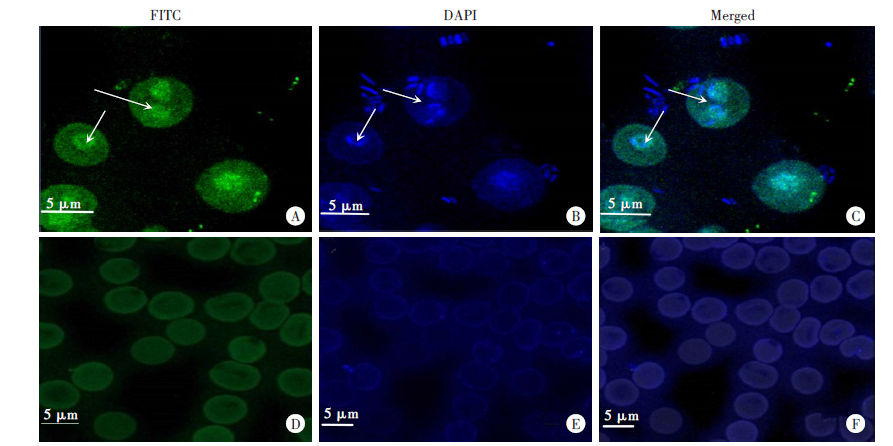
Fig. 4
Immunofluorescence location of BmPPIase in red blood cells of mice infected with B. microti A-C: Red blood cells of B. microti infection mice, the infected red blood cells are pseudopod-like protrusions with different sizes. D-F: Red blood cells of healthy mice, the red blood cells are in the shape of double-concave discs, with smooth surface and uniform size.
| [1] |
Vial HJ, Gorenflot A. Chemotherapy against babesiosis[J]. Vet Parasitol, 2006, 138(1/2): 147-160.
doi: 10.1016/j.vetpar.2006.01.048 |
| [2] | Zhou X, Wang H, Xue JB, et al. Epidemic and research progress of babesiosis[J]. Chin J Schisto Control, 2019, 31(1): 63-70. (in Chinese) |
| (周霞, 王慧, 薛靖波, 等. 国内外巴贝虫病流行现状与研究进展[J]. 中国血吸虫病防治杂志, 2019, 31(1): 63-70.) | |
| [3] |
Schnittger L, Rodriguez AE, Florin-Christensen M, et al. Babesia: a world emerging[J]. Infect Genet Evol, 2012, 12(8):1788-1809.
doi: 10.1016/j.meegid.2012.07.004 pmid: 22871652 |
| [4] |
Goethert HK. What Babesia microti is now[J]. Pathogens, 2021, 10(9): 1168.
doi: 10.3390/pathogens10091168 |
| [5] | Song P, Cai YC, Lu Y, et al. Establishment of mouse infection model of Babesia microti Lishui isolate and consequent pathological changes[J]. Chin J Parasitol Parasit Dis, 2022, 40(4): 493-499.. (in Chinese) |
| (宋鹏, 蔡玉春, 卢艳, 等. 田鼠巴贝虫丽水分离株小鼠感染模型的建立及其病理变化[J]. 中国寄生虫学与寄生虫病杂志, 2022, 40(4): 493-499.) | |
| [6] | Yao LN, Ruan W, Zeng CY, et al. Pathogen identification and clinical diagnosis for one case infected with Babesia[J]. Chin J Parasitol Parasit Dis, 2012, 30(2): 118-121. (in Chinese) |
| (姚立农, 阮卫, 曾长佑, 等. 1例人感染巴贝虫的诊断与病原体鉴定[J]. 中国寄生虫学与寄生虫病杂志, 2012, 30(2): 118-121.) | |
| [7] |
Bloch EM, Kumar S, Krause PJ. Persistence of Babesia microti infection in humans[J]. Pathogens, 2019, 8(3): 102.
doi: 10.3390/pathogens8030102 |
| [8] |
Krause PJ. Human babesiosis[J]. Int J Parasitol, 2019, 49(2): 165-174.
doi: S0020-7519(19)30005-0 pmid: 30690090 |
| [9] |
Wittner M, Rowin KS, Tanowitz HB, et al. Successful chemotherapy of transfusion babesiosis[J]. Ann Intern Med, 1982, 96(5): 601-604.
pmid: 7200341 |
| [10] |
Krause PJ, Lepore T, Sikand VK, et al. Atovaquone and azithromycin for the treatment of babesiosis[J]. N Engl J Med, 2000, 343(20): 1454-1458.
doi: 10.1056/NEJM200011163432004 |
| [11] | Yin M, Zhang HB, Tao Y, et al. Evaluation on the in vivo efficacy of malarone and atovaquoneazithromycin combination against Babesia microti in mice under different immune status[J]. Chin J Parasitol Parasit Dis, 2021, 39(5): 659-665, 673. (in Chinese) |
| (殷梦, 张皓冰, 陶奕, 等. 马拉龙和阿托伐醌 + 阿奇霉素在不同免疫状态小鼠体内的抗田鼠巴贝虫药效评价[J]. 中国寄生虫学与寄生虫病杂志, 2021, 39(5): 659-665, 673.) | |
| [12] |
Wormser GP, Prasad A, Neuhaus E, et al. Emergence of resistance to azithromycin-atovaquone in immunocompromised patients with Babesia microti infection[J]. Clin Infect Dis, 2010, 50(3): 381-386.
doi: 10.1086/649859 pmid: 20047477 |
| [13] |
Shaw PE. Peptidyl-prolyl cis/trans isomerases and transcription: is there a twist in the tail?[J]. EMBO Rep, 2007, 8(1): 40-45.
pmid: 17203101 |
| [14] |
Schiene-Fischer C. Multidomain peptidyl prolyl cis/trans isomerases[J]. Biochim Biophys Acta, 2015, 1850(10): 2005-2016.
doi: 10.1016/j.bbagen.2014.11.012 pmid: 25445709 |
| [15] |
Solbach W, Forberg K, Kammerer E, et al. Suppressive effect of cyclosporin A on the development of Leishmania tropica-induced lesions in genetically susceptible BALB/c mice[J]. J Immunol, 1986, 137(2): 702-707.
pmid: 3487578 |
| [16] |
Bell A, Wernli B, Franklin RM. Roles of peptidyl-prolyl cis-trans isomerase and calcineurin in the mechanisms of antimalarial action of cyclosporin A, FK506, and rapamycin[J]. Biochem Pharmacol, 1994, 48(3): 495-503.
pmid: 7520696 |
| [17] |
Perrone AE, Milduberger N, Fuchs AG, et al. A functional analysis of the cyclophilin repertoire in the protozoan parasite Trypanosoma cruzi[J]. Biomolecules, 2018, 8(4): 132.
doi: 10.3390/biom8040132 |
| [18] |
Krause PJ, McKay K, Gadbaw J, et al. Increasing health burden of human babesiosis in endemic sites[J]. Am J Trop Med Hyg, 2003, 68(4): 431-436.
pmid: 12875292 |
| [19] | Zhou X, Xia S, Huang JL, et al. Human babesiosis, an emerging tick-borne disease in the People’s Republic of China[J]. Parasit Vectors, 2014, 7(1): 509. |
| [20] |
Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America[J]. Clin Infect Dis, 2006, 43(9): 1089-1134.
doi: 10.1086/508667 pmid: 17029130 |
| [21] |
Sanchez E, Vannier E, Wormser GP, et al. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: a review[J]. JAMA, 2016, 315(16): 1767-1777.
doi: 10.1001/jama.2016.2884 pmid: 27115378 |
| [22] |
Harikishore A, Yoon HS. Immunophilins: structures, mechanisms and ligands[J]. Curr Mol Pharmacol, 2015, 9(1): 37-47.
pmid: 25986569 |
| [23] |
Liu J, Farmer JD Jr, Lane WS, et al. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes[J]. Cell, 1991, 66(4): 807-815.
doi: 10.1016/0092-8674(91)90124-h pmid: 1715244 |
| [24] |
Gavigan CS, Kiely SP, Hirtzlin J, et al. Cyclosporin-binding proteins of Plasmodium falciparum[J]. Int J Parasitol, 2003, 33(9): 987-996.
pmid: 12906882 |
| [25] |
Ibrahim HM, Xuan XN, Nishikawa Y. Toxoplasma gondii cyclophilin 18 regulates the proliferation and migration of murine macrophages and spleen cells[J]. Clin Vaccine Immunol, 2010, 17(9): 1322-1329.
doi: 10.1128/CVI.00128-10 pmid: 20660134 |
| [26] | Guo ZY, Jing CX, Huang XY, et al. The bioinformatic analysis of CyPs from Cryptosporidium parvum[J]. J Trop Med, 2018, 18(10): 1263-1269. (in Chinese) |
| (郭志云, 荆春霞, 黄小英, 等. 微小隐孢子虫CyPs家族蛋白的生物信息学分析[J]. 热带医学杂志, 2018, 18(10): 1263-1269.) | |
| [27] |
Potenza M, Galat A, Minning TA, et al. Analysis of the Trypanosoma cruzi cyclophilin gene family and identification of Cyclosporin A binding proteins[J]. Parasitology, 2006, 132(Pt 6): 867-882.
pmid: 16700961 |
| [28] |
Krücken J, Greif G, von Samson-Himmelstjerna G. In silico analysis of the cyclophilin repertoire of apicomplexan parasites[J]. Parasit Vectors, 2009, 2(1): 27.
doi: 10.1186/1756-3305-2-27 pmid: 19555495 |
| [29] |
Deng WW, Wang L, Xiong Y, et al. The novel secretory protein CGREF1 inhibits the activation of AP-1 transcriptional activity and cell proliferation[J]. Int J Biochem Cell Biol, 2015, 65: 32-39.
doi: 10.1016/j.biocel.2015.05.019 pmid: 26022276 |
| [30] |
Liao YT, Luo D, Peng KL, et al. Cyclophilin A: a key player for etiological agent infection[J]. Appl Microbiol Biotechnol, 2021, 105(4): 1365-1377.
doi: 10.1007/s00253-021-11115-2 pmid: 33492451 |
| [31] |
Zhang Y, Jiang N, Lu HJ, et al. Proteomic analysis of Plasmodium falciparum schizonts reveals heparin-binding merozoite proteins[J]. J Proteome Res, 2013, 12(5): 2185-2193.
doi: 10.1021/pr400038j pmid: 23566259 |
| [1] | SONG Peng, CAI Yu-chun, LU Yan, AI Lin, CHEN Mu-xin, CHEN Shao-hong, CHEN Jia-xu. Establishment of mouse infection model of Babesia microti Lishui isolate and consequent pathological changes [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(4): 493-499. |
| [2] | YIN Meng, ZHANG Hao-bing, TAO Yi, JIANG Bin, LIU Hua. Evaluation on the in vivo efficacy of malarone and atovaquone-azithromycin combination against Babesia microti in mice under different immune status [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(5): 659-665. |
| [3] | ZHANG Yan, XU Ai-fang, ZHANG Jia-qi, YAO Li-nong, GU Kai-long, XUE Li-zhi, PAN Ke-nv. Differential diagnosis of a case of Babesia microti infection previously misdiagnosed as malaria [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2020, 38(4): 445-448. |
| [4] | Zi-yue WANG, Yi-chao YANG, Zhi-pin CHEN, Yun-liang SHI. Infection of Plasmodium knowlesi and Babesia microti in farmed monkeys in Guangxi [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2019, 37(4): 494-496. |
| [5] | Su-hua LI, Yu-ling ZHAO, Li-jun GAO, Ya-lan ZHANG, Rui-min ZHOU, Dan QIAN, Cheng-yun YANG, Ying LIU, De-ling LU, Hong-wei ZHANG, Bian-li XU. Analysis of molecular epidemiology of babesiosis in patients having fever and thrombocytopenia in Xinyang City, Henan Province [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2019, 37(1): 66-69. |
| [6] | CAI Yu-chun, CHEN Shao-hong, LI Hao, LU Yan, AI Lin, CHU Yan-hong, . Mass spectrometry identification and bioinformatics analysis of soluble proteins of Babesia microti [J]. , 2018, 36(3): 8-246-252. |
| [7] | Xiu-feng LIU, Jia-hui SUN, Bin XU, Jun-hu CHEN, Wei HU. Expression and evaluation of diagnostic candidate antigens from Babesia microti [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2017, 35(6): 549-553. |
| [8] | CAI Yu-chun1,2, CHEN Shao-hong2, LU Yan2, AI Lin2, YANG Chun-li2, CHEN Jia-xu2*. Dynamic changes of density of Babesia microti in mice with latent infection after re-infection, immunosuppression, or random transmission to healthy mice [J]. , 2017, 35(4): 4-327-332. |
| [9] | Wei RUAN, Ling-ling ZHANG, Hua-liang CHEN, Qiao-yi LU, Xuan ZHANG, Yan FENG, Li-nong YAO. Investigation of the source regions of Babesia spp. infection in the central and south areas of Zhejiang Province [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2017, 35(2): 125-130. |
| [10] | Fang-zhen XIAO, Xiu-qing Peng, Guo-ying XU, Yang CHEN, Dai-hua LIN, Yan-qin DENG. Investigation and genetic identification on Babesia infection in rodents in some areas of Fujian Province [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2017, 35(1): 63-67. |
| [11] | JIANG Shi-chen, WEI Hai-xia, HE Cheng, DENG Sheng-qun, XIA Jing, PENG Hong-juan*. Secretion and Distribution of Rhoptry Protein 16 during Toxoplasma gondii Invasion into Host Cells [J]. , 2016, 34(3): 2-189-197. |
| [12] | LU Zhi-min1,WANG Yan1,ZHANG Zi-yang2,TANG Hong-wei1,SUO Xun3 *. Evaluation of an Indirect Immunofluorescence Assay Kit for the Dectetion of Anti-Toxoplasma gondii IgG [J]. , 2013, 31(5): 4-346-351. |
| [13] | TIANYong-hong;XIONGCheng-liang;GUANHuang-tao;PANGXue-bing;JIANGChang-fu. Detection of Trichomonas vaginalis with Direct Immunofluorescence Assay [J]. , 2003, 21(4): 14-244. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||