
CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES ›› 2021, Vol. 39 ›› Issue (6): 746-752.doi: 10.12140/j.issn.1000-7423.2021.06.004
• ORIGINAL ARTICLES • Previous Articles Next Articles
YU Jia-li1( ), LIU Lei2, YANG Bo1, CHU Rui-lin3, SUN Yi-fan1, LIU Yao-bao4, CHENG Yang1,*(
), LIU Lei2, YANG Bo1, CHU Rui-lin3, SUN Yi-fan1, LIU Yao-bao4, CHENG Yang1,*( )
)
Received:2021-03-15
Revised:2021-05-06
Online:2021-12-30
Published:2021-12-15
Contact:
CHENG Yang
E-mail:18328089416@163.com;woerseng@126.com
Supported by:CLC Number:
YU Jia-li, LIU Lei, YANG Bo, CHU Rui-lin, SUN Yi-fan, LIU Yao-bao, CHENG Yang. Antigenicity and immunogenicity analysis of the recombinant merozoite surface protein 1 N-terminal of Plasmodium ovale[J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(6): 746-752.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.jsczz.cn/EN/10.12140/j.issn.1000-7423.2021.06.004
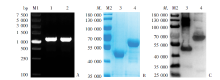
Fig. 1
Construction of PoMSP1 N-terminal plasmid and validation of protein expression A: Pomsp1 N terminal gene amplified by PCR; B: Expression of recombinant proteins by SDS-PAGE; C: Expression of recombinant proteins by Western blotting. M1: DNA marker; M2: Protein marker. 1: Pocmsp1 N-terminal; 2: Powmsp1 N-terminal; 3: Purified rPocMSP1 N-terminal; 4: Purified rPowMSP1 N-terminal.
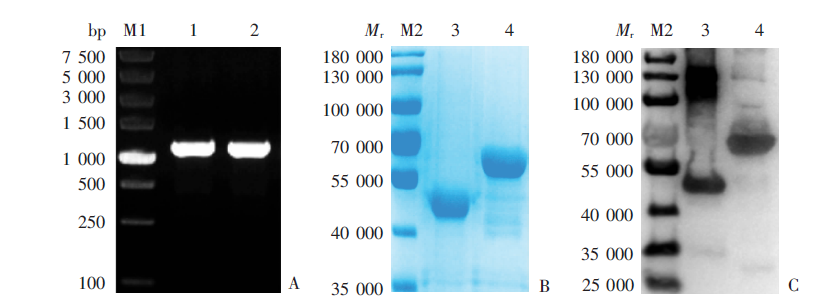
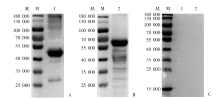
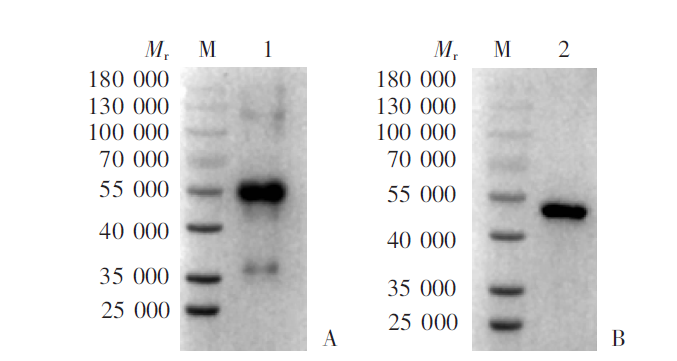
Fig. 2
Western blotting detection for the specificity of serum IgG antibody of mice immunized with rPocMSP1 N-terminal and rPowMSP1 N-terminal protein A: rPocMSP1 N-terminal protein-immunized group; B: rPowMSP1 N-terminal protein-immunized group; C: PBS group. M: Protein marker; 1: purified rPocMSP1 N-terminal protein; 2: purified rPowMSP1 N-terminal protein.
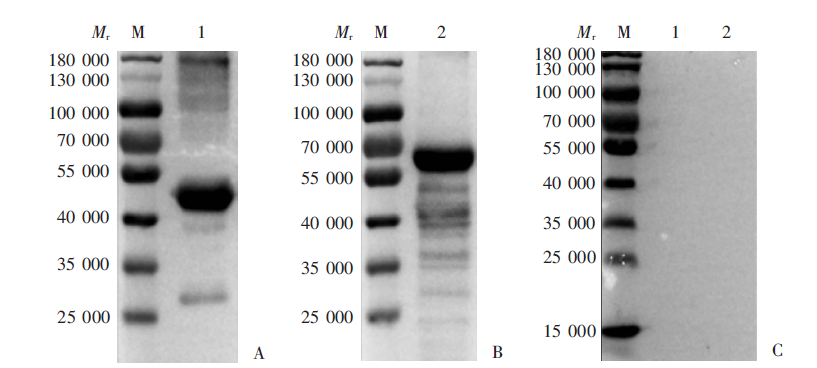
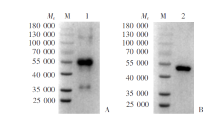
Fig. 3
Cross-reactivity of serum from mice immunized with rPocMSP1 N-terminal and rPowMSP1 N-terminal protein detected by Western blotting A: rPocMSP1 N-terminal protein-immunized group; B: rPowMSP1 N-terminal protein-immunized group. M: Protein marker; 1: Purified rPowMSP1 N-terminal protein; 2: purified rPocMSP1 N-terminal protein.
| [1] | World Health Organization. World Malaria Report 2021[R]. Geneva: World Health Organization, 2021. |
| [2] | Zhang L, Feng J, Tu H, et al. Malaria epidemiology in China in 2020[J]. Chin J Parasitol Parasit Dis, 2021, 39(2): 195-199. (in Chinese) |
| (张丽, 丰俊, 涂宏, 等. 2020年全国疟疾疫情分析[J]. 中国寄生虫学与寄生虫病杂志, 2021, 39(2): 195-199.) | |
| [3] | Feng J, Zhou SS. From control to elimination: the historical retrospect of malaria control and prevention in China[J]. Chin J Parasitol Parasit Dis, 2019, 37(5): 505-513. (in Chinese) |
| (丰俊, 周水森. 从控制走向消除: 我国疟疾防控的历史回顾[J]. 中国寄生虫学与寄生虫病杂志, 2019, 37(5): 505-513.) | |
| [4] | Wang J, Shen Y, Li Y, et al. Recent progress in immune checkpoint molecules in Plasmodium infection and immunity[J]. Chin J Parasitol Parasit Dis, 2019, 37(4): 472-480. (in Chinese) |
| (王军, 沈燕, 李悦, 等. 免疫检查点分子调控在疟原虫感染与免疫中的研究进展[J]. 中国寄生虫学与寄生虫病杂志, 2019, 37(4): 472-480.) | |
| [5] |
Baldwin MR, Li X, Hanada T, et al. Merozoite surface protein 1 recognition of host glycophorin a mediates malaria parasite invasion of red blood cells[J]. Blood, 2015, 125(17): 2704-2711.
doi: 10.1182/blood-2014-11-611707 |
| [6] |
Holder AA. The precursor to major merozoite surface antigens: structure and role in immunity[J]. Prog Allergy, 1988, 41: 72-97.
pmid: 3043424 |
| [7] |
Blackman MJ, Holder AA. Secondary processing of the Plasmodium falciparum merozoite surface protein-1 (MSP1) by a calcium-dependent membrane-bound serine protease: shedding of MSP133 as a noncovalently associated complex with other fragments of the MSP1[J]. Mol Biochem Parasitol, 1992, 50(2): 307-315.
doi: 10.1016/0166-6851(92)90228-C |
| [8] |
Thái TL, Jun H, Lee J, et al. Genetic diversity of merozoite surface protein-1 C-terminal 42 kDa of Plasmodium falciparum (PfMSP-142) may be greater than previously known in global isolates[J]. Parasit Vectors, 2018, 11(1): 455.
doi: 10.1186/s13071-018-3027-x pmid: 30081943 |
| [9] |
Soares IS, Levitus G, Souza JM, et al. Acquired immune responses to the N- and C-terminal regions of Plasmodium vivax merozoite surface protein 1 in individuals exposed to malaria[J]. Infect Immun, 1997, 65(5): 1606-1614.
doi: 10.1128/iai.65.5.1606-1614.1997 pmid: 9125537 |
| [10] | Versiani FG, Almeida ME, Mariuba LA, et al. N-terminal Plasmodium vivax merozoite surface protein-1, a potential subunit for malaria vivax vaccine[J]. Clin Dev Immunol, 2013, 2013: 965841. |
| [11] |
Elizardez YB, Fotoran WL, Junior AJG, et al. Recombinant proteins of Plasmodium malariae merozoite surface protein 1 (PmMSP1): testing immunogenicity in the BALB/c model and potential use as diagnostic tool[J]. PLoS One, 2019, 14(7): e0219629.
doi: 10.1371/journal.pone.0219629 |
| [12] |
Chu R, Zhang X, Xu S, et al. Limited genetic diversity of N-terminal of merozoite surface protein-1 (MSP-1) in Plasmodium ovale curtisi and P. ovale wallikeri imported from Africa to China[J]. Parasit Vectors, 2018, 11(1): 596.
doi: 10.1186/s13071-018-3174-0 |
| [13] | Chen J, Liu YB, Tang F, et al. Polymorphism analysis of K13 gene of Plasmodium ovale isolates from Africa[J]. Chin J Parasitol Parasit Dis, 2019, 37(2): 167-172. (in Chinese) |
| (陈静, 刘耀宝, 唐凤, 等. 卵形疟原虫非洲分离株K13基因的多态性分析[J]. 中国寄生虫学与寄生虫病杂志, 2019, 37(2): 167-172.) | |
| [14] | Peng H, Wang YY, Zhou AG, et al. Enhancement of immunogenicity of Plasmodium falciparum antigens by combination of CpG ODN protein with montanide ISA720 adjuvant[J]. Chin Trop Med, 2010, 10(7): 786-788. (in Chinese) |
| (彭恒, 王颖玉, 周爱国, 等. ISA720与CpG联合佐剂增强恶性疟原虫抗原的免疫原性[J]. 中国热带医学, 2010, 10(7): 786-788.) | |
| [15] |
Mamillapalli A, Sunil S, Diwan SS, et al. Polymorphism and epitope sharing between the alleles of merozoite surface protein-1 of Plasmodium falciparum among Indian isolates[J]. Malar J, 2007, 6: 95.
doi: 10.1186/1475-2875-6-95 |
| [16] | Takala SL, Coulibaly D, Thera MA,, et al. Extreme polymorphism in a vaccine antigen and risk of clinical malaria: implications for vaccine development[J]. Sci Transl Med, 2009, 1(2): 2ra5. |
| [17] |
Duffy PE, Patrick GJ. Malaria vaccines since 2000: progress, priorities, products[J]. Npj Vaccines, 2020, 5(1): 1-9.
doi: 10.1038/s41541-019-0151-3 |
| [18] |
Read AF, Baigent SJ, Powers C, et al. Imperfect vaccination can enhance the transmission of highly virulent pathogens[J]. PLoS Biol, 2015, 13(7): e1002198.
doi: 10.1371/journal.pbio.1002198 |
| [19] | Qian F, Xu HJ. Malaria conjugate vaccine[J]. Chin J Parasitol Parasit Dis, 2012, 30(5): 393-395, 400. (in Chinese) |
| (钱锋, 徐沪济. 疟疾偶联疫苗[J]. 中国寄生虫学与寄生虫病杂志, 2012, 30(5): 393-395, 400.) | |
| [20] |
Chen JT, Li J, Zha GC, et al. Genetic diversity and allele frequencies of Plasmodium falciparum msp1 and msp2 in parasite isolates from Bioko Island, Equatorial Guinea[J]. Malar J, 2018, 17: 458.
doi: 10.1186/s12936-018-2611-z |
| [21] | Soares LA, Evangelista J, Orlandi PP, et al. Genetic diversity of MSP1 Block 2 of Plasmodium vivax isolates from Manaus (central Brazilian Amazon)[J]. J Immunol Res, 2014, 2014: 671050. |
| [22] |
Noranate N, Prugnolle F, Jouin H, et al. Population diversity and antibody selective pressure to Plasmodium falciparum MSP1 block 2 locus in an African malaria-endemic setting[J]. BMC Microbiol, 2009, 9: 219.
doi: 10.1186/1471-2180-9-219 |
| [23] |
Cheng Y, Wang B, Sattabongkot J, et al. Immunogenicity and antigenicity of Plasmodium vivax merozoite surface protein 10[J]. Parasitol Res, 2014, 113(7): 2559-2568.
doi: 10.1007/s00436-014-3907-8 pmid: 24764159 |
| [1] | LU Xing, WANG Shuiyi, CHEN Linjun, LIU Mingming, LIU Yutong, ZHU Huiru, JIANG Bingbing, DU Shaolei, BAYIN Chahan, LIU Dandan, ZHANG Wei. Cloning and prokaryotic expression of Theileria equi rhoptry neck protein 5 gene [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(4): 497-501. |
| [2] | ZHANG Yao-guang, JIANG Li, WANG Zhen-yu, ZHU Min, ZHU Qian, MA Xiao-Jiang, WU Huan-yu. Laboratory diagnosis of two misdiagnosed imported Plasmodium ovale malaria cases in Shanghai [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(4): 553-556. |
| [3] | Bei JIANG, Xiao-jun XIAO, Chun-yan OUYANG, Xin-ping LUO, Bao-qing SUN, Jing LI, Zhi-gang LIU. Cloning and expression of must mite allergen Der f 32 and its activation on mouse dendritic cells [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2019, 37(3): 279-285. |
| [4] | Jing CHEN, Yao-bao LIU, Feng TANG, Feng LU, Jian-xia TANG, Jun CAO. Polymorphism analysis of K13 gene of Plasmodium ovale isolates from Africa [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2019, 37(2): 167-172. |
| [5] | Ling-ling ZHANG, Li-nong YAO, Hua-liang CHEN, Qiao-yi LU, Wei RUAN. Single nucleotide polymorphism analysis of cytochrome b, cytochrome c oxidaseⅠand lactate dehydrogenase genes of imported Plasmodium ovale [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2017, 35(5): 429-433. |
| [6] | Xi-shuai JIA, Shui-mao ZHOU, Yan YANG, Ming-xing XU, Kai WU. Genotyping of merozoite surface protein 1 and 2 of imported Plasmodium falciparum in Wuhan City [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2017, 35(5): 434-439. |
| [7] | LIU Guang-xue1, ZHANG Shao-hua1, GUO Ai-jiang1,2, HOU Jun-ling1, WEI Yan-ling1, WANG Shuai1, LUO Xue-nong1,2*. Identification, Expression and Antigenicity Analysis of Serpin B6 of Taenia solium [J]. , 2016, 34(4): 7-334-338,345. |
| [8] | ZHAO Yin-qi1,2,3,LI Zi-hua1,2,3,WANG Hao2,4,ZHU Ming-xing1,2,3,NIU Nan2,3,5,. Cloning, Expression and Immungenicity Analysis of Antigen Eg-01883 Screened from Protoscoleces of Echinococcus granulosus [J]. , 2016, 34(3): 5-208-214. |
| [9] | WANG Yan-juan, CAO Jian-ping, SUN Ya-wen, XU Yu-xin, SHEN Yu-juan* . Expression of Cocktail DNA Vaccine Comprising Toxoplasma gondii SAGl, MIC3 and ROP2 Using Fluorescent Protein-Reporting Vectors and Evaluation of Its Immunogenicity [J]. , 2015, 33(5): 8-368--371. |
| [10] | WANG Zhi-sheng,WU Jing,LIN Yuan,LI Hong-bing,LIU Qiang,WANG Zhi-hui,LANG Duo-yong,YANG Wen*. Construction and Immunogenicity Analysis of the Attenuated Recombinant Salmonella typhimurium Strains Expressing Echinococcus granulosus Eg95 Antigen [J]. , 2014, 32(5): 3-339-343. |
| [11] | YANG Meng-jia1,WANG Su-rong1,ZHUGE Hong-xiang1 *,CHEN Jun-hu2. Cloning,Expression and Antigenic Analysis of Merozoite Surface Protein MSPDBL2-DBL2 Domain from Plasmodium falciparum [J]. , 2014, 32(5): 7-357-360. |
| [12] | DAN Xu-Hua, WEN Xiao-Bei-*, WANG Chun-Ren, LI Xiao-Juan, WEI Xiao-Man. Immunoreactivity and Immunogenicity Analysis of the Recombinant Cathepsin L-like Protease of Fasciola hepatica in SD Rats [J]. , 2014, 32(4): 9-289-292. |
| [13] | LI Xing-Pan, CAO Guo-Mei, XING Cui-Cui, HUA Qian-Qian, ZHANG Liang-Liang, LIANG Shao-Hui. Preparation and Antigenicity Analysis of Recombinant Aegyptin-like Protein of Aedes albopictus [J]. , 2014, 32(3): 6-193-197. |
| [14] | LIU Zhuan-zhuan1,2,YANG Yan-ping1,2,YIN Guo-rong 2 *,WANG Hai-long 2,. Cloning,Expression and Immunogenicity Analysis of Malate Dehydrogenase Gene of Toxoplasma gondii [J]. , 2013, 31(1): 3-12-17. |
| [15] | HUANG Jiang1, 2,LI Bo1,DAI Jia-lin1,ZHANG Ai-hua2 *. Prokaryotic Expression and Histolocalization of the Taenia solium CDC37 Gene [J]. , 2013, 31(1): 5-23-26. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||