
CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES ›› 2023, Vol. 41 ›› Issue (4): 397-403.doi: 10.12140/j.issn.1000-7423.2023.04.001
• ORIGINAL ARTICLES • Previous Articles Next Articles
WANG Zhiqian( ), WANG Jingwen, SONG Xiumei*(
), WANG Jingwen, SONG Xiumei*( )
)
Received:2022-07-22
Revised:2022-10-17
Online:2023-08-30
Published:2023-09-06
Contact:
*E-mail: Supported by:CLC Number:
WANG Zhiqian, WANG Jingwen, SONG Xiumei. Function analysis of Anopheles stephensi peptidoglycan recognition protein S2 in regulating homeostasis of symbiotic microbiota[J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(4): 397-403.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.jsczz.cn/EN/10.12140/j.issn.1000-7423.2023.04.001
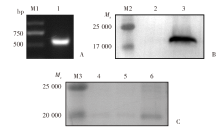
Fig. 3
Cloning of pgrp-s2 gene, and expression and purification of PGRP-S2 A: pgrp-s2 gene; B: Western blotting of PGRP-S2 recombinant protein; C: Purified PGRP-S2 recombinant protein. M1: DNA marker; 1: pgrp-s2 gene; M2, M3: Protein marker; 2: Negative control, 3: PGRP-S2 recombinant protein; 4-6: Purified PGRP-S2 recombinant protein.
| [1] | WHO. World malaria report 2022[J]. Geneva: World Health Organization, 2022: 14-17. |
| [2] |
Wang SB, Jacobs-Lorena M. Genetic approaches to interfere with malaria transmission by vector mosquitoes[J]. Trends Biotechnol, 2013, 31(3): 185-193.
doi: 10.1016/j.tibtech.2013.01.001 pmid: 23395485 |
| [3] |
Singh M, Suryanshu, Kanika, et al. Plasmodium's journey through the Anopheles mosquito: a comprehensive review[J]. Biochimie, 2021, 181: 176-190.
doi: 10.1016/j.biochi.2020.12.009 |
| [4] |
Dziarski R. Peptidoglycan recognition proteins (PGRPs)[J]. Mol Immunol, 2004, 40(12): 877-886.
pmid: 14698226 |
| [5] |
Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity[J]. Cell, 2006, 124(4): 783-801.
doi: 10.1016/j.cell.2006.02.015 pmid: 16497588 |
| [6] |
Werner T, Liu G, Kang D, et al. A family of peptidoglycan recognition proteins in the fruit fly Drosophila melanogaster[J]. Proc Natl Acad Sci USA, 2000, 97(25): 13772-13777.
pmid: 11106397 |
| [7] |
Liu C, Xu Z, Gupta D, et al. Peptidoglycan recognition proteins: a novel family of four human innate immunity pattern recognition molecules[J]. J Biol Chem, 2001, 276(37): 34686-34694.
doi: 10.1074/jbc.M105566200 pmid: 11461926 |
| [8] |
Kim MS, Byun M, Oh BH. Crystal structure of peptidoglycan recognition protein LB from Drosophila melanogaster[J]. Nat Immunol, 2003, 4(8): 787-793.
doi: 10.1038/ni952 |
| [9] |
Mellroth P, Karlsson J, Steiner H. A scavenger function for a Drosophila peptidoglycan recognition protein[J]. J Biol Chem, 2003, 278(9): 7059-7064.
doi: 10.1074/jbc.M208900200 pmid: 12496260 |
| [10] |
Gao L, Song XM, Wang JW. Gut microbiota is essential in PGRP-LA regulated immune protection against Plasmodium berghei infection[J]. Parasit Vectors, 2020, 13(1): 3.
doi: 10.1186/s13071-019-3876-y pmid: 31907025 |
| [11] |
Kaneko T, Yano T, Aggarwal K, et al. PGRP-LC and PGRP-LE have essential yet distinct functions in the drosophila immune response to monomeric DAP-type peptidoglycan[J]. Nat Immunol, 2006, 7(7): 715-723.
pmid: 16767093 |
| [12] |
Zaidman-Rémy A, Hervé M, Poidevin M, et al. The Drosophila amidase PGRP-LB modulates the immune response to bacterial infection[J]. Immunity, 2006, 24(4): 463-473.
pmid: 16618604 |
| [13] |
Wang JW, Aksoy S. PGRP-LB is a maternally transmitted immune milk protein that influences symbiosis and parasitism in tsetse's offspring[J]. Proc Natl Acad Sci USA, 2012, 109(26): 10552-10557.
doi: 10.1073/pnas.1116431109 pmid: 22689989 |
| [14] |
Mendes C, Felix R, Sousa AM, et al. Molecular evolution of the three short PGRPs of the malaria vectors Anopheles gambiae and Anopheles arabiensis in East Africa[J]. BMC Evol Biol, 2010, 10: 9.
doi: 10.1186/1471-2148-10-9 |
| [15] |
Feng YB, Peng YQ, Song XM, et al. Anopheline mosquitoes are protected against parasite infection by tryptophan catabolism in gut microbiota[J]. Nat Microbiol, 2022, 7(5): 707-715.
doi: 10.1038/s41564-022-01099-8 pmid: 35437328 |
| [16] |
Holmes DS, Bonner J. Preparation, molecular weight, base composition, and secondary structure of giant nuclear ribonucleic acid[J]. Biochemistry, 1973, 12(12): 2330-2338.
pmid: 4710584 |
| [17] |
Yang H, Li XX, Song WJ, et al. Involvement of a short-type peptidoglycan recognition protein (PGRP) from Chinese giant salamanders Andrias davidianus in the immune response against bacterial infection[J]. Dev Comp Immunol, 2018, 88: 37-44.
doi: S0145-305X(18)30227-1 pmid: 30017855 |
| [18] |
Cirimotich CM, Dong YM, Garver LS, et al. Mosquito immune defenses against Plasmodium infection[J]. Dev Comp Immunol, 2010, 34(4): 387-395.
doi: 10.1016/j.dci.2009.12.005 pmid: 20026176 |
| [19] |
Bahuguna S, Atilano M, Glittenberg M, et al. Bacterial recognition by PGRP-SA and downstream signalling by Toll/DIF sustain commensal gut bacteria in Drosophila[J]. PLoS Genet, 2022, 18(1): e1009992.
doi: 10.1371/journal.pgen.1009992 |
| [20] |
Li C, Hong PP, Yang MC, et al. FoxO regulates the expression of antimicrobial peptides and promotes phagocytosis of hemocytes in shrimp antibacterial immunity[J]. PLoS Pathog, 2021, 17(4): e1009479.
doi: 10.1371/journal.ppat.1009479 |
| [21] |
Wang JW, Song XM, Wang MF. Peptidoglycan recognition proteins in hematophagous arthropods[J]. Dev Comp Immunol, 2018, 83: 89-95.
doi: S0145-305X(17)30516-5 pmid: 29269264 |
| [22] | Song XM, Wang JW. Influence of nutritional metabolism of Anopheles on its transmission capability of malaria parasites[J]. Chin J Parasitol Parasit Dis, 2021, 39(5): 617-620. (in Chinese) |
| (宋秀梅, 王敬文. 营养代谢对按蚊传播疟原虫能力的影响[J]. 中国寄生虫学与寄生虫病杂志, 2021, 39(5): 617-620.) | |
| [23] |
Connors J, Dawe N, Van Limbergen J. The role of succinate in the regulation of intestinal inflammation[J]. Nutrients, 2018, 11(1): 25.
doi: 10.3390/nu11010025 |
| [24] |
Guo LL, Karpac J, Tran SL, et al. PGRP-SC2 promotes gut immune homeostasis to limit commensal dysbiosis and extend lifespan[J]. Cell, 2014, 156(1/2): 109-122.
doi: 10.1016/j.cell.2013.12.018 |
| [1] | FAN Jun-jie, HAN Xiu-min, Nur Fazleen Binti Idris, LI Kai, TAN Qing-qing, CAO Wen-qiao, LI Xiang, LIAO Peng, YE Bin. Bioinformatics characteristics and immunoreactivity of protein kinase A of Echinococcus granulosus [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2020, 38(6): 682-687. |
| [2] | Yu-mei XU, Shi-de CAO, Chuan-gang ZHU, Shi-qing ZHANG. Expression of fusion protein of Sj28GST epitopes and cholera toxin B subunit in baculovirus/insect cells [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2019, 37(3): 364-367. |
| [3] | PU Jue-biao1,2,TANG Li-li3,YIN Cen-nan1,DU Xin-yue1, WU Jian-hua1, ZHAO Wei1, . Recombinant expression of calcium-binding protein from Clonorchis sinensis and preliminary evaluation of its application in immunodiagnosis [J]. , 2018, 36(3): 12-275-279. |
| [4] | GUO Ling-ling,ZHANG Xiao-lei,ZHANG Jin-shun*,JIA Xiao-hui,WANG Chun-miao,. Construction of Eukaryotic Expression Vector Containing ROP18-ROP12 of Toxoplasma gondii RH Strain [J]. , 2015, 33(3): 1-161-166. |
| [5] | WANG Hai-long1,YIN Li-tian2,ZHANG Tie-e1,GUAN Li1,3,MENG Xiao-li1,LIU Hong-li1,YIN Guo-rong1 *. Construction,Expression and Kinase Function Analysis of an Eukaryocyte Vector of Rhoptry Protein 17 in Toxoplasma gondii [J]. , 2014, 32(1): 6-29-33. |
| [6] | SUN Min, HE Shen-Yi, ZHAO An-Hui, CONG Hua, ZHOU Huai-Yu, ZHAO Qun-Li, MENG Min. Construction and Expression of an Eukaryocyte Vector of 14-3-3 Protein in Toxoplasma gondii [J]. , 2012, 30(6): 5-438-441. |
| [7] | ZHANGJian;XUWen-yue;DUANJian-hua;HUANGFu-sheng*. Correlation of Anopheles TEP1 Gene with Melanization Induced by Nitroquine [J]. , 2009, 27(4): 7-325. |
| [8] | ZHENGMei-juan;LIMin;WANGZhi-cheng;LUOFei;LUOQing-li;CHUDe-yong;LICong-lei;SHENJi-long. Secreted Expression of Signaling Protein 14-3-3 of Schistosoma japanicom in Pichia pastoris System with Primary Evaluation on its Antigenicity [J]. , 2007, 25(1): 3-16. |
| [9] | HUYong-xuan;XIAOJian-hua;HUANGJia-fang;YANGQiu-lin. Construction of Sjcb2 DNA Vaccine and its Expression in HeLa Cells [J]. , 2006, 24(5): 16-386. |
| [10] | CUIJing;WANGZhongquan*;HANHuamin;WEIHaiyan;ZHANGHongwei;LIYonglong. Expression of DNA Vaccine against Trichinella spiralis in Chinese Hamster Ovary Cells [J]. , 2004, 22(5): 3-270. |
| [11] | CUIJing;ZHANGHong-wei;WANGZhong-quan;LIYong-long. Construction of DNA Vaccine of Trichinella spiralis Muscle Larvae and Its Expression in Mice [J]. , 2004, 22(1): 2-8. |
| [12] | MiaoJun;XueCaifang;YuQigui;QinEnqiang. CONSTRUCTION OF HYBRID NUCLEIC ACID VACCINES BASED ON PLASMODIUM FALCIPARUM MEROZOITE SURFACE PROTEIN1BLOCK17REGION [J]. , 1997, 15(6): 321-325. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||