
CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES ›› 2022, Vol. 40 ›› Issue (2): 168-174.doi: 10.12140/j.issn.1000-7423.2022.02.006
• ORIGINAL ARTICLES • Previous Articles Next Articles
GAO Yuan( ), ZHANG Xiao-cheng, HU Yuan*(
), ZHANG Xiao-cheng, HU Yuan*( ), CAO Jian-ping
), CAO Jian-ping
Received:2021-08-10
Revised:2021-10-20
Online:2022-04-16
Published:2022-04-16
Contact:
HU Yuan
E-mail:gyuan1028@126.com;huyuan@nipd.chinacdc.cn
Supported by:CLC Number:
GAO Yuan, ZHANG Xiao-cheng, HU Yuan, CAO Jian-ping. Study on the inhibitory effect of natural killer cells on liver fibrosis of schistosomiasis[J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(2): 168-174.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.jsczz.cn/EN/10.12140/j.issn.1000-7423.2022.02.006
Table 1
Primer sequences for qPCR
| 基因名称Gene name | 引物序列(5′→3′) Primer sequence (5′→3′) |
|---|---|
| β-肌动蛋白 β-actin | F: CATTGCTGACAGGATGCAGAAGG R: TGCTGGAAGGTGGACAGTGAGG |
| Ⅰ型胶原蛋白 Ⅰ-collagen | F: CCTCAGGGTATTGCTGGACAAC R: CAGAAGGACCTTGTTTGCCAGG |
| Ⅲ型胶原蛋白 Ⅲ-collagen | F: GACCAAAAGGTGATGCTGGACAG R: CAAGACCTCGTGCTCCAGTTAG |
| Ⅳ型胶原蛋白 Ⅳ-collagen | F: ATGGCTTGCCTGGAGAGATAGG R: TGGTTGCCCTTTGAGTCCTGGA |
| 纤维连接蛋白Fibronectin | F: GGTCCTCTCCTTCCATCTCCTTAC R: GGACCCCTGAGCATCTTGAGTG |
| 层粘连蛋白 Laminin | F: GAACTACACGGTGAGGTTGGAG R: GCCAACAGTGAAGATGTCCAGC |
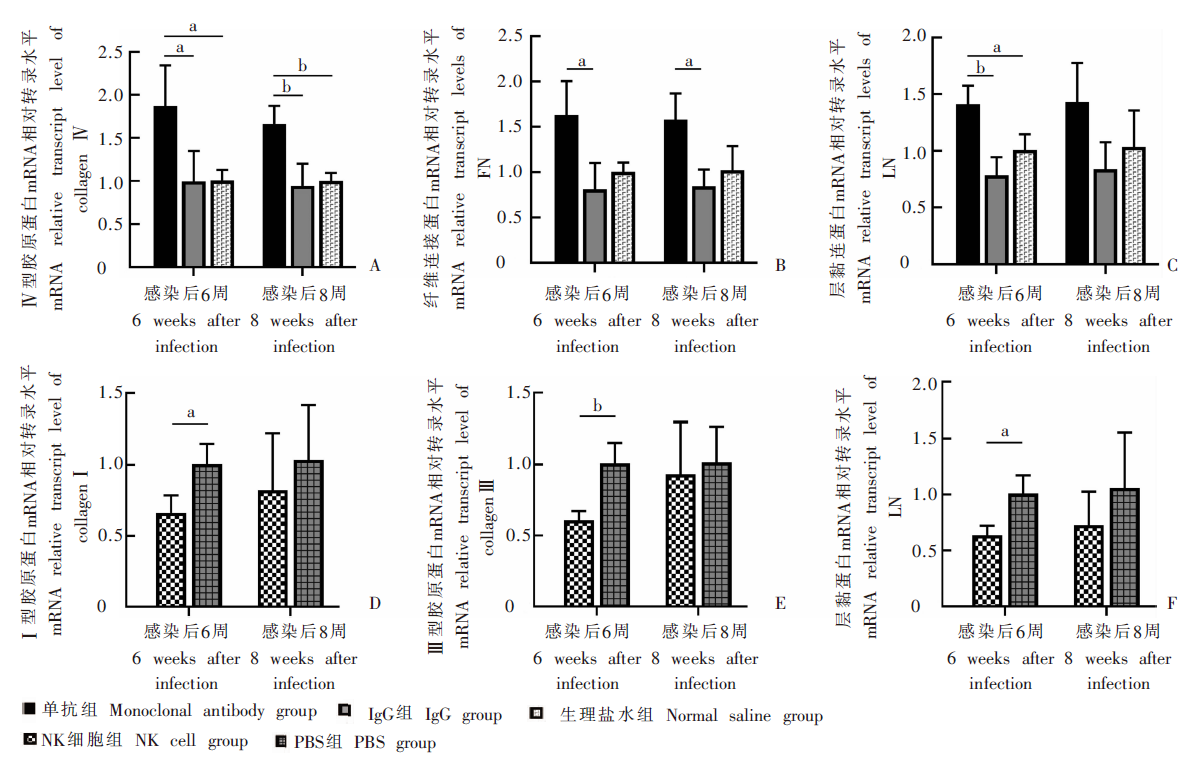
Fig. 3
Changes of mRNA relative transcription levels for liver fibrosis-related proteins in infected mice for different treatment groups A-C: mRNA relative transcription levels for Ⅳ-C、FN and LN at 6 and 8 weeks after infection in the monoclonal antibody group, IgG group and normal saline group; D-F: mRNA relative transcript levels for Ⅰ-C、Ⅲ-C and LN at 6 and 8 weeks after infection in the NK cell group and PBS group. a: P < 0.05; b: P < 0.01.
| [1] |
McManus DP,, Bergquist R,, Cai PF, et al. Schistosomiasis-from immunopathology to vaccines[J]. Semin Immunopathol, 2020, 42(3): 355-371.
doi: 10.1007/s00281-020-00789-x pmid: 32076812 |
| [2] | Zhang LJ,, Xu ZM,, Yang F, et al. Endemic status of schistosomiasis in People’s Republic of China in 2020[J]. Chin J Schisto Control, 2021, 33(3): 225-233. (in Chinese) |
| (张利娟,, 徐志敏,, 杨帆, 等. 2020年全国血吸虫病疫情通报[J]. 中国血吸虫病防治杂志, 2021, 33(3): 225-233.) | |
| [3] |
Wang L,, Wang YH,, Quan J. Exosomes derived from natural killer cells inhibit hepatic stellate cell activation and liver fibrosis[J]. Hum Cell, 2020, 33(3): 582-589.
doi: 10.1007/s13577-020-00371-5 |
| [4] |
van Eeden C,, Khan L,, Osman MS, et al. Natural killer cell dysfunction and its role in COVID-19[J]. Int J Mol Sci, 2020, 21(17): 6351.
doi: 10.3390/ijms21176351 |
| [5] |
Wijaya RS,, Read SA,, Schibeci S, et al. KLRG1+ natural killer cells exert a novel antifibrotic function in chronic hepatitis B[J]. J Hepatol, 2019, 71(2): 252-264.
doi: S0168-8278(19)30182-5 pmid: 30905683 |
| [6] |
Yi HS,, Lee YS,, Byun JS, et al. Alcohol dehydrogenase Ⅲ exacerbates liver fibrosis by enhancing stellate cell activation and suppressing natural killer cells in mice[J]. Hepatology, 2014, 60(3): 1044-1053.
doi: 10.1002/hep.27137 |
| [7] |
Fan YT,, Zhang WD,, Wei HM, et al. Hepatic NK cells attenuate fibrosis progression of non-alcoholic steatohepatitis in dependent of CXCL10-mediated recruitment[J]. Liver Int, 2020, 40(3): 598-608.
doi: 10.1111/liv.14307 |
| [8] |
Hou X,, Yu FZ,, Man SQ, et al. Negative regulation of Schistosoma japonicum egg-induced liver fibrosis by natural killer cells[J]. PLoS Negl Trop Dis, 2012, 6(1): e1456.
doi: 10.1371/journal.pntd.0001456 |
| [9] |
Villesen IF,, Daniels SJ,, Leeming DJ, et al. Review article: the signalling and functional role of the extracellular matrix in the development of liver fibrosis[J]. Aliment Pharmacol Ther, 2020, 52(1): 85-97.
doi: 10.1111/apt.15773 |
| [10] | Jiang PY,, Pan WQ. Advances in liver fibrosis and pathogenesis of schistosomiasis[J]. Chin Trop Med, 2018, 18(8): 847-852. (in Chinese) |
| (姜鹏月,, 潘卫庆. 血吸虫病肝纤维化及其致病机制研究进展[J]. 中国热带医学, 2018, 18(8): 847-852.) | |
| [11] |
Hu Y,, Wang XL,, Wei YH, et al. Functional inhibition of natural killer cells in a BALB/c mouse model of liver fibrosis induced by Schistosoma japonicum infection[J]. Front Cell Infect Microbiol, 2020, 10: 598987.
doi: 10.3389/fcimb.2020.598987 |
| [12] |
Terrén I,, Orrantia A,, Vitallé J, et al. NK cell metabolism and tumor microenvironment[J]. Front Immunol, 2019, 10: 2278.
doi: 10.3389/fimmu.2019.02278 pmid: 31616440 |
| [13] |
Becker PSA,, Suck G,, Nowakowska P, et al. Selection and expansion of natural killer cells for NK cell-based immunotherapy[J]. Cancer Immunol Immunother, 2016, 65(4): 477-484.
doi: 10.1007/s00262-016-1792-y pmid: 26810567 |
| [14] |
Peng H,, Tian ZG. NK cells in liver homeostasis and viral hepatitis[J]. Sci China Life Sci, 2018, 61(12): 1477-1485.
doi: 10.1007/s11427-018-9407-2 pmid: 30421296 |
| [15] |
Fasbender F,, Widera A,, Hengstler JG, et al. Natural killer cells and liver fibrosis[J]. Front Immunol, 2016, 7: 19.
doi: 10.3389/fimmu.2016.00019 pmid: 26858722 |
| [16] | Hayakawa Y,, Andrews DM,, Smyth MJ. Subset analysis of human and mouse mature NK cells[J]. Methods Mol Biol, 2010, 612: 27-38. |
| [17] |
Widowati W,, Jasaputra DK,, Sumitro SB, et al. Effect of interleukins (IL-2, IL-15, IL-18) on receptors activation and cytotoxic activity of natural killer cells in breast cancer cell[J]. Afr Health Sci, 2020, 20(2): 822-832.
doi: 10.4314/ahs.v20i2.36 pmid: 33163049 |
| [18] |
Liu C,, Zhang YS,, Chen F, et al. Immunopathology in schistosomiasis is regulated by TLR2, 4- and IFN-γ-activated MSC through modulating Th1/Th2 responses[J]. Stem Cell Res Ther, 2020, 11(1): 217.
doi: 10.1186/s13287-020-01735-2 |
| [19] |
Chester C,, Fritsch K,, Kohrt HE. Natural killer cell immunomodulation: targeting activating, inhibitory, and co-stimulatory receptor signaling for cancer immunotherapy[J]. Front Immunol, 2015, 6: 601.
doi: 10.3389/fimmu.2015.00601 pmid: 26697006 |
| [20] |
Khomich O,, Ivanov AV,, Bartosch B. Metabolic hallmarks of hepatic stellate cells in liver fibrosis[J]. Cells, 2019, 9(1): 24.
doi: 10.3390/cells9010024 |
| [21] |
Hamidzadeh K,, Christensen SM,, Dalby E, et al. Macrophages and the recovery from acute and chronic inflammation[J]. Annu Rev Physiol, 2017, 79: 567-592.
doi: 10.1146/annurev-physiol-022516-034348 pmid: 27959619 |
| [22] | Zhang YX,, Xiong DH,, Li YY, et al. Schistosoma japonicum infection in treg-specific USP21 knockout mice[J]. J Immunol Res, 2021, 2021: 6613162. |
| [23] |
Hammerich L,, Tacke F. Emerging roles of myeloid derived suppressor cells in hepatic inflammation and fibrosis[J]. World J Gastrointest Pathophysiol, 2015, 6(3): 43-50.
doi: 10.4291/wjgp.v6.i3.43 pmid: 26301117 |
| [24] |
Park HS,, Kim J,, Cho MY, et al. Effectual labeling of natural killer cells with upconverting nanoparticles by electroporation for in vivo tracking and biodistribution assessment[J]. ACS Appl Mater Interfaces, 2020, 12(44): 49362-49370.
doi: 10.1021/acsami.0c12849 |
| [1] | TAN Xiao, ZHU Qi, LIU Zhongqi, LI Jia, PENG Dingjin. Immunogenicity of Schistosoma japonicum Sj26gst mRNA vaccine candidate [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(5): 546-551. |
| [2] | LIU Huaman, Bikash Giri, FANG Chuantao, ZHENG Yameng, WU Huixin, ZENG Minhao, LI Shan, CHENG Guofeng. Identification of gender associated m6A modified circRNA in Schistosoma japonicum [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(5): 552-558. |
| [3] | LAN Weiming, XU Hui, XU Yin, QIU Tingting, XIE Shuying, DENG Fenglin, HU Shaoliang, LIU Huan, GUO Jiagang, ZENG Xiaojun. Study on early warning of high risk environment of Schistosoma japonicum infection by quantitative real-time PCR [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(4): 502-505. |
| [4] | LI Tianxing, ZHANG Jiaming, XU Chenxi, WANG Zige, GUO Jingjie, LI Shan. Mechanism of a Chinese patent medicine in the treatment of liver fibrosis caused by infection of Clonorchis sinensis based on network pharmacology [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(4): 510-515. |
| [5] | WANG Xiao-ling, ZHANG Wei, YI Cun, CHEN Xiang-yu, YANG Wen-bin, XU Bin, HU Wei. The effect of SjGPR89 protein on the growth and development of Schistosoma japonicum [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(6): 701-707. |
| [6] | CHEN Guo, ZHU Dan-dan, DUAN Yi-nong. Research progress of immune regulation protein B7 family on immune regulation during Schistosoma japonicum infection [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(6): 774-779. |
| [7] | YAN Xiao-lan, WEN Li-yong, XIONG Yan-hong, ZHENG Bin, ZHANG Jian-feng, WANG Tian-ping, YU Li-ling, XU Guo-zhang, LIN Dan-dan, ZHOU Xiao-nong. Interpretation of Criteria for Detection of Antibody against Schistosoma japonicum—Enzyme-linked Immunosorbent Assay [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(6): 798-800. |
| [8] | TANG Xian-shi, JI Wen-xiang, XIONG Chun-rong, ZHOU Yong-hua, XU Yong-liang, TONG De-sheng. Study on anxiety-like behavior of mice with late-stage infection of Schistosoma japonicum [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(5): 622-628. |
| [9] | LIANG Le, ZHANG Jing, SHEN Yu-juan, HU Yuan, CAO Jian-ping. Cyclic guanosine monophosphate-adenosine monophosphate promotes liver egg granuloma formation and fibrosis in mice infected with Schistosoma japonicum [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(4): 441-445. |
| [10] | ZHANG Ya-lan, JIANG Tian-tian, HE Zhi-quan, DENG Yan, CHEN Wei-qi, ZHU Yan-kun, ZHANG Hong-wei, ZHAO Dong-yang. Differential expression of microRNA in the liver of mice infected by Capillaria hepatica [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(1): 56-60. |
| [11] | GAO Yuan, HU Yuan, CAO Jian-ping. Research progress on the role of immune cells in liver fibrosis due to schistosomiasis [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(1): 88-93. |
| [12] | HONG Yang, LIN Jiao-jiao. Research progress on proteomics in Schistosoma japonicum [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(6): 725-730. |
| [13] | MA Wen-mei, SANG Wei, AIMAITI Ya-sen, ZUO Li-ke, FU Li, MIAO Na. Roles of nuclear factor-κB/myeloid differentiation factor 88 in the liver fibrosis of cystic echinococcosis patients [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(6): 779-783. |
| [14] | HUANG Ai-long, ZHANG Bei, SHEN Han-yu, CHEN Guo, LI Jing, ZHU Dan-dan, DUAN Yi-nong. Expression and function of triggering receptor expressed on myeloid cells 1 in the liver of mice infected with Schistosoma japonicum [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(5): 621-626. |
| [15] | LI Wen-ding, WEN Hao, HOU Jiao, WANG Ming-kun, LI liang, LI Jing, ZHANG Chuan-shan, SUN Bing, WANG Hui. Role of extracellular matrix protein 1 in the liver fibrosis induced by Echinococcus multilocularis infection in mice [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(3): 296-303. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||