
CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES ›› 2022, Vol. 40 ›› Issue (1): 28-35.doi: 10.12140/j.issn.1000-7423.2022.01.004
• ORIGINAL ARTICLES • Previous Articles Next Articles
CHEN Sui-lin( ), GAO Yuan-li, GUO Shuai, FAN Yong-ling, LIU Tai-ping, XU Wen-yue*(
), GAO Yuan-li, GUO Shuai, FAN Yong-ling, LIU Tai-ping, XU Wen-yue*( )
)
Received:2021-12-02
Revised:2021-12-21
Online:2022-02-28
Published:2022-01-24
Contact:
XU Wen-yue
E-mail:chensuilin0208@163.com;xuwenyue@tmmu.edu.cn
Supported by:CLC Number:
CHEN Sui-lin, GAO Yuan-li, GUO Shuai, FAN Yong-ling, LIU Tai-ping, XU Wen-yue. Effect and mechanism of high-dose clodronate liposomes treatment on Plasmodium yoelii growth in mice[J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(1): 28-35.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.jsczz.cn/EN/10.12140/j.issn.1000-7423.2022.01.004

Fig. 2
The production of Plasmodium-specific antibody and the activation of Plasmodium-specific CD4+ T cells of infected mice treated with high-dose clodronate liposomes (CL) A:The mice serum IgG antibody level on day 6 after infection;B:The capacity of Plasmodium-specific CD4+ T cell secreting IFN-γ;C:The capacity of Plasmodium-specific CD4+ T cell proliferation. a: P < 0.05, b: P < 0.01.
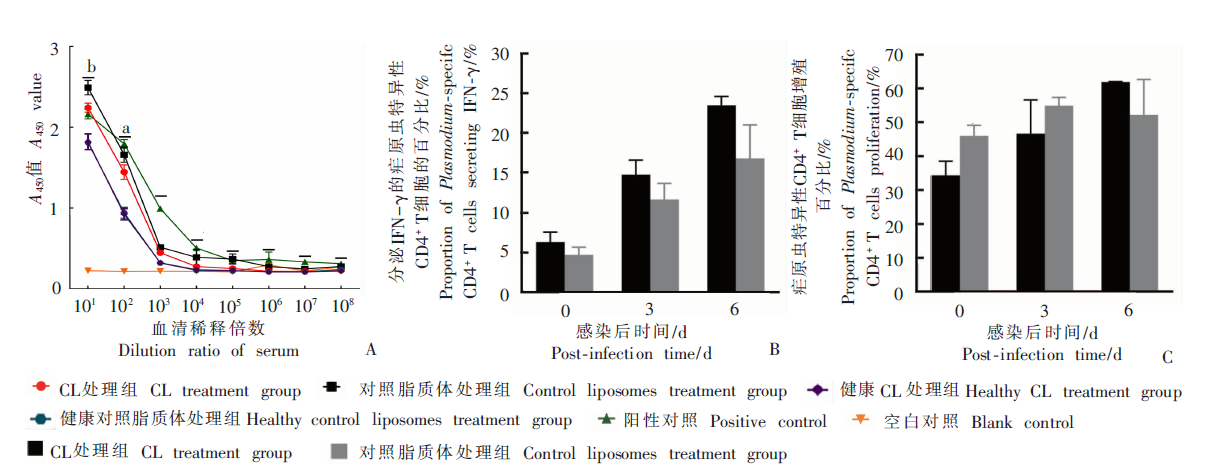

Fig. 3
The production of Plasmodium-specific antibody and the activation of Plasmodium-specific CD8+ T cells of infected mice treated with high-dose clodronate liposomes (CL) A:The capacity of Plasmodium-specific CD8+ T cells secreting IFN-γ;B:The proliferationcapacity of Plasmodium-specific CD8+ T cell.
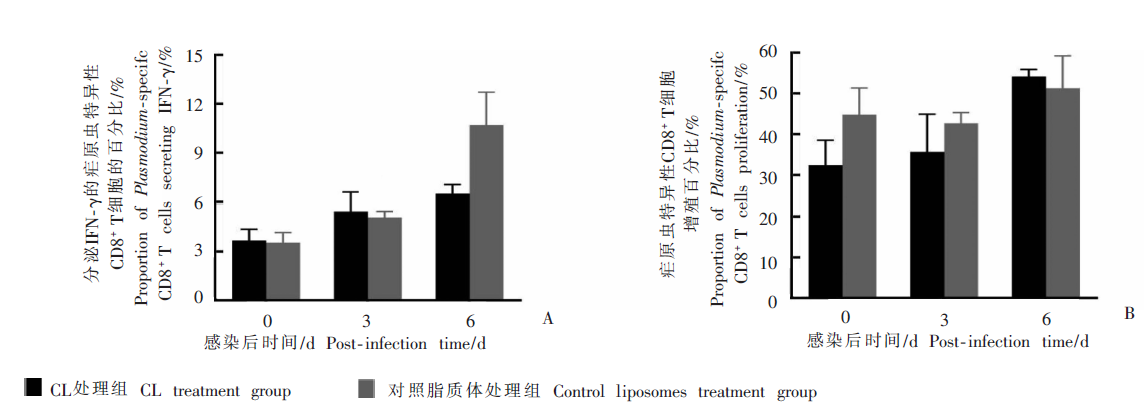
Fig. 4
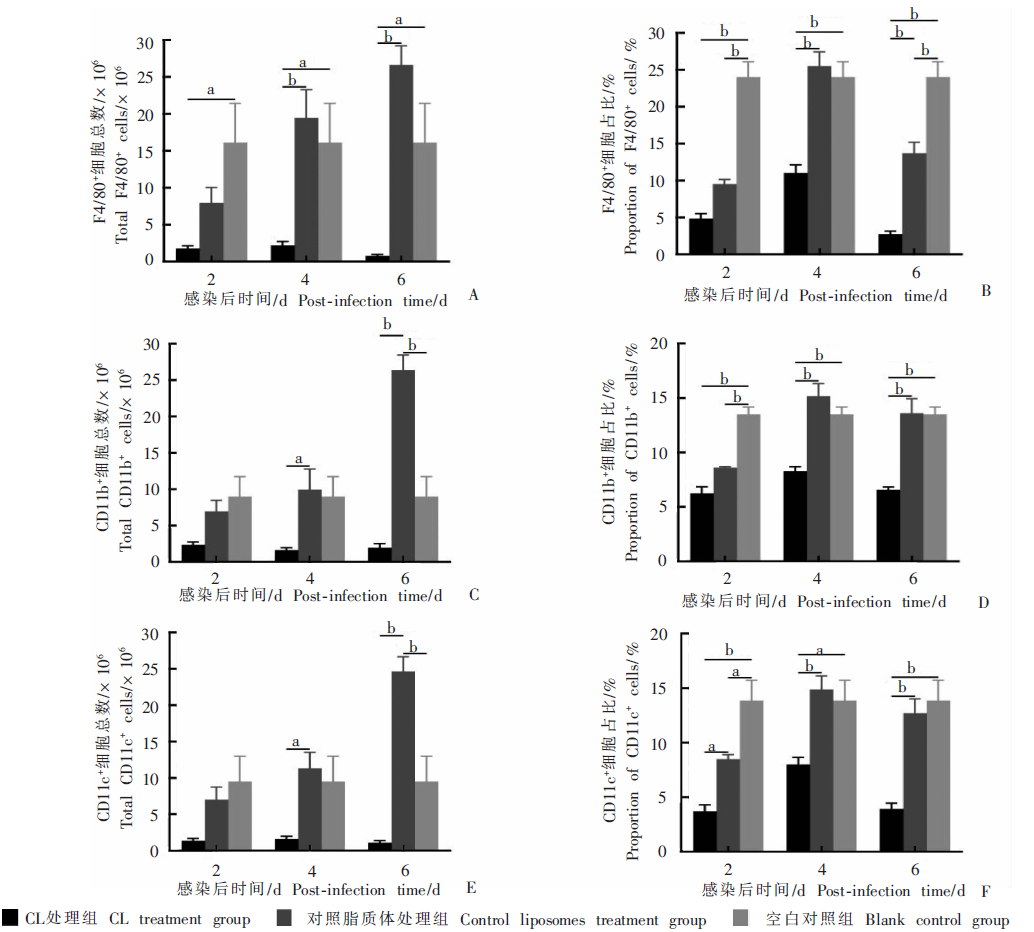
Effects on splenic lymphocytes in infected mice treated with high-dose clodronate liposomes (CL) A:Total number of F4/80+ cells;B:Proportion of F4/80+ cells;C:Total number of CD11b+ cells;D:Proportion of CD11b+ cells;E:Total number of CD11c+ cells;F:Proportion of CD11c+ cells. a: P < 0.05; b: P < 0.01.
| [1] | WHO. Approves historic RTS,S malaria vaccine[EB/OL].(2021-10-07)[2021-12-01]. https://lenstapesmedcom/who-approves-historic-rtss-malaria-vaccine/.2021. |
| [2] |
RTS, S Clinical Trials Partnership, Agnandji ST, Lell B, et al. First results of phase 3 trial of RTS, S/AS01 malaria vaccine in African children[J]. N Engl J Med, 2011, 365(20): 1863-1875.
doi: 10.1056/NEJMoa1102287 |
| [3] |
RTS, S Clinical Trials Partnership, Agnandji ST, Lell B, et al. A phase 3 trial of RTS, S/AS01 malaria vaccine in African infants[J]. N Engl J Med, 2012, 367(24): 2284-2295.
doi: 10.1056/NEJMoa1208394 |
| [4] |
Jain A, Pasare C. Innate control of adaptive immunity: beyond the three-signal paradigm[J]. J Immunol, 2017, 198(10): 3791-3800.
doi: 10.4049/jimmunol.1602000 |
| [5] |
Schofield L, Grau GE. Immunological processes in malaria pathogenesis[J]. Nat Rev Immunol, 2005, 5(9): 722-735.
pmid: 16138104 |
| [6] | van Rooijen N, van Kesteren-Hendrikx E. “In vivo” depletion of macrophages by liposome-mediated “suicide”[J]. Methods Enzymol, 2003, 373: 3-16. |
| [7] |
Borges da Silva H, Fonseca R, Cassado A, et al. In vivo approaches reveal a key role for DCs in CD4+ T cell activation and parasite clearance during the acute phase of experimental blood-stage malaria[J]. PLoS Pathog, 2015, 11(2): e1004598.
doi: 10.1371/journal.ppat.1004598 |
| [8] |
Grabowska J, Lopez-Venegas MA, Affandi AJ, et al. CD169+ macrophages capture and dendritic cells instruct: the interplay of the gatekeeper and the general of the immune system[J]. Front Immunol, 2018, 9: 2472.
doi: 10.3389/fimmu.2018.02472 pmid: 30416504 |
| [9] |
Sponaas AM, Freitas do Rosario AP, Voisine C, et al. Migrating monocytes recruited to the spleen play an important role in control of blood stage malaria[J]. Blood, 2009, 114(27): 5522-5531.
doi: 10.1182/blood-2009-04-217489 |
| [10] |
Gupta P, Lai SM, Sheng JP, et al. Tissue-resident CD169+ macrophages form a crucial front line against Plasmodium infection[J]. Cell Rep, 2016, 16(6): 1749-1761.
doi: 10.1016/j.celrep.2016.07.010 |
| [11] |
Meding SJ, Langhorne J. CD4+ T cells and B cells are necessary for the transfer of protective immunity to Plasmodium chabaudi chabaudi[J]. Eur J Immunol, 1991, 21(6): 1433-1438.
pmid: 1675172 |
| [12] |
Su Z, Stevenson MM. Central role of endogenous gamma interferon in protective immunity against blood-stage Plasmodium chabaudi AS infection[J]. Infect Immun, 2000, 68(8): 4399-4406.
doi: 10.1128/IAI.68.8.4399-4406.2000 pmid: 10899836 |
| [13] | Inoue SI, Niikura M, Mineo S, et al. Roles of IFN-γ and γδ T cells in protective immunity against blood-stage malaria[J]. Front Immunol, 2013, 4: 258. |
| [14] |
Salles ÉM, Menezes MN, Siqueira R, et al. P2X7 receptor drives Th1 cell differentiation and controls the follicular helper T cell population to protect against Plasmodium chabaudi malaria[J]. PLoS Pathog, 2017, 13(8): e1006595.
doi: 10.1371/journal.ppat.1006595 |
| [15] |
Cheng QQ, Liu J, Pei YJ, et al. Neddylation contributes to CD4+ T cell-mediated protective immunity against blood-stage Plasmodium infection[J]. PLoS Pathog, 2018, 14(11): e1007440.
doi: 10.1371/journal.ppat.1007440 |
| [16] |
Zander RA, Vijay R, Pack AD, et al. Th1-like Plasmodium-specific memory CD4+ T cells support humoral immunity[J]. Cell Rep, 2017, 21(7): 1839-1852.
doi: S2211-1247(17)31540-1 pmid: 29141217 |
| [17] |
Kurup SP, Butler NS, Harty JT. T cell-mediated immunity to malaria[J]. Nat Rev Immunol, 2019, 19(7): 457-471.
doi: 10.1038/s41577-019-0158-z |
| [18] |
Kurup SP, Butler NS, Harty JT. T cell-mediated immunity to malaria[J]. Nat Rev Immunol, 2019, 19(7): 457-471.
doi: 10.1038/s41577-019-0158-z |
| [19] |
Stephens R, Albano FR, Quin S, et al. Malaria-specific transgenic CD4+ T cells protect immunodeficient mice from lethal infection and demonstrate requirement for a protective threshold of antibody production for parasite clearance[J]. Blood, 2005, 106(5): 1676-1684.
doi: 10.1182/blood-2004-10-4047 pmid: 15890689 |
| [20] |
Oakley MS, Sahu BR, Lotspeich-Cole L, et al. T-bet modulates the antibody response and immune protection during murine malaria[J]. Eur J Immunol, 2014, 44(9): 2680-2691.
doi: 10.1002/eji.201344437 |
| [21] |
Perez-Mazliah D, Nguyen MP, Hosking C, et al. Follicular helper T cells are essential for the elimination of Plasmodium infection[J]. EBioMedicine, 2017, 24: 216-230.
doi: 10.1016/j.ebiom.2017.08.030 |
| [22] |
Junqueira C, Barbosa CRR, Costa PAC, et al. Cytotoxic CD8+ T cells recognize and kill Plasmodium vivax-infected reticulocytes[J]. Nat Med, 2018, 24(9): 1330-1336.
doi: 10.1038/s41591-018-0117-4 pmid: 30038217 |
| [23] |
Imai T, Ishida H, Suzue K, et al. Cytotoxic activities of CD8+ T cells collaborate with macrophages to protect against blood-stage murine malaria[J]. elife, 2015, 4: e04232.
doi: 10.7554/eLife.04232 |
| [24] |
Wykes MN, Kay JG, Manderson A, et al. Rodent blood-stage Plasmodium survive in dendritic cells that infect native mice[J]. Proc Natl Acad Sci USA, 2011, 108(27): 11205-11210.
doi: 10.1073/pnas.1108579108 |
| [1] | GUO Shuai, HE Biao, GAO Yuanli, FAN Yongling, ZHU Feng, DING Yan, LIU Taiping, XU Wenyue. Specie-specific analysis of plasmodia infecting rats and mice [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(5): 539-545. |
| [2] | YE Jingming, HE Wei, LIU Huiyuan, YU Xiao, LUO Bo, LIU Meichen, ZHOU Biying. Effect of excretory-secretory antigen TPx of Cysticercus cellulosae on activation of dendritic cells in piglets [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(3): 286-293. |
| [3] | ZHANG Ling-hui, CHEN Gen, CHONG Shi-gui, SHEN Hui, MA Hui, ZHAO Yu-min. Research progress on the immune regulation mechanism in alveolar echinococcosis [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2022, 40(1): 109-113. |
| [4] | DU Kai-ge, ZHUO Xun-hui, LU Shao-hong. Research advances on the innate immunity mechanisms against Toxoplasma gondii [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2020, 38(6): 764-770. |
| [5] | WANG Zuo-ling, PAN Yan-yan, SUN Xiao-dan, CAO Ya-ming. Effect of PD-1 blockade on immune responses in mice infected with Plasmodium berghei [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2020, 38(4): 405-411. |
| [6] | YU Xiao-dong, YALI Ya-sen, WANG Jia-ling, LI Meng, YE Jian-rong. Establishment of BALB/c mouse model of Echinococcus granulosus-induced sensitization and changes of related immune cells [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2020, 38(4): 412-416. |
| [7] | Xiang-yun CHENG, Tai-ping LIU, Sui-lin CHEN, Wen-yue XU. Study on the role of protein kinase 9 in the growth and development of Plasmodium yoelii [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2019, 37(3): 248-253. |
| [8] | Bei JIANG, Xiao-jun XIAO, Chun-yan OUYANG, Xin-ping LUO, Bao-qing SUN, Jing LI, Zhi-gang LIU. Cloning and expression of must mite allergen Der f 32 and its activation on mouse dendritic cells [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2019, 37(3): 279-285. |
| [9] | Shang-hua WU, E-yan GENG, Jing ZHANG, Heng ZHANG, Zhi-qiang SHI, Shan WANG, Wei LU, Yi-zhen WU, Gui-jun WANG, Yong WANG, Qian-ming XU. Effects of recombinant autophagy related 5 protein on the maturation of dendritic cells stimulated by Toxoplasma gondii [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2018, 36(5): 464-468. |
| [10] | Yong FU, Ru MENG, Hai-fang XUE, Hai-ning FAN, Hai-feng NIU, Zi-jia ZHOU, Hong-bin WANG. Morphological observation and phenotypic detection of dendritic cells from peripheral blood of patients with alveolar echinococcosis [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2018, 36(5): 474-477. |
| [11] | Fen FANG, Zhe LIU, Gui-jun WANG, Qian-ming XU. In vivo characterization of mouse dendritic cells infected with Cryptosporidium parvum in the presence of Toll-like receptor 4 [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2017, 35(3): 265-269. |
| [12] | Xiao-jie LIU, Tie-bo MAO, Rui ZHOU. The role of innate immunity in defending against infection with Cryptosporidium parvum [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2017, 35(2): 185-192. |
| [13] | Guang CHEN, Lei LIU, Fang-fang WANG, Sheng BI, Lan LUO, Ju-xiang SU, Hui-ming ZHANG, Lian-shun CAI, Zi-lin GONG. The regulatory effect of dendritic cells on Th17 cell differentiation and function in mouse infected with Plasmodium yoelii [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2017, 35(1): 8-12. |
| [14] | LIU Bo-yu, WANG Cheng, XING Xin, CHEN Hong-liang, JIANG Jing, CAI Ya-nan,WANG Chun-feng, YANG Gui-lian*. Dynamic Changes of Dectin-2 Expression on Dendritic Cells in Mice Infected with Trichinella spiralis [J]. , 2016, 34(2): 3-105-108. |
| [15] | JIANG Jing1,2,ZHAO Quan2,YANG Gui-lian1 *. The Role of Dendritic Cells in Host Immunity against Helminth Infections [J]. , 2015, 33(2): 16-147-150. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||