
CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES ›› 2021, Vol. 39 ›› Issue (4): 455-460.doi: 10.12140/j.issn.1000-7423.2021.04.006
• ORIGINAL ARTICLES • Previous Articles Next Articles
LI Shu-ning1( ), LI Wen-lin1, SHEN Hai-e2, WANG Yang2,*(
), LI Wen-lin1, SHEN Hai-e2, WANG Yang2,*( ), TIAN Xi-feng3
), TIAN Xi-feng3
Received:2021-03-15
Revised:2021-06-19
Online:2021-08-30
Published:2021-08-05
Contact:
WANG Yang
E-mail:924058540@qq.com;konig718@163.com
Supported by:CLC Number:
LI Shu-ning, LI Wen-lin, SHEN Hai-e, WANG Yang, TIAN Xi-feng. Giardia lamblia trophozoites induce the formation of neutrophil extracellular traps in vitro[J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(4): 455-460.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.jsczz.cn/EN/10.12140/j.issn.1000-7423.2021.04.006
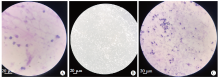
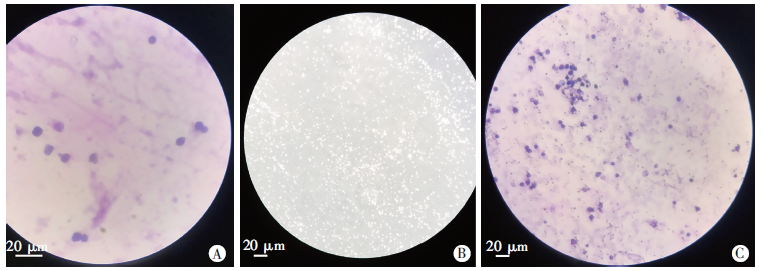
Fig. 1
Morphology of Giardia Lamblia trophozoites and neutrophils observed by light microscopy A: The nuclei of neutrophils were deeply stained, curved, rod-shaped or lobulated (Gram staining, × 400); B: Giardia lamblia trophozoites were present in an inverted-pear shape with active movement (× 200); C: After neutrophils and G. Lamblia trophozoites were co-cultured, neutrophils showed a distribution pattern of aggregation and the Giardia trophozoites were inactive (Gram staining, × 200).
| [1] |
Adam RD. Biology of Giardia lamblia[J]. Clin Microbiol Rev, 2001, 14(3):447-475.
pmid: 11432808 |
| [2] |
Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria[J]. Science, 2004, 303(5663):1532-1535.
pmid: 15001782 |
| [3] | Rada B. Neutrophil extracellular traps[J]. Methods Mol Biol Clifton N J, 2019, 1982:517-528. |
| [4] |
Mori YK, Yamaguchi M, Terao Y, et al. α-Enolase of Streptococcus pneumonia induces formation of neutrophil extracellular traps[J]. J Biol Chem, 2012, 287(13):10472-10481.
doi: 10.1074/jbc.M111.280321 |
| [5] |
Abi Abdallah DS, Lin CY, Ball CJ, et al. Toxoplasma gondii triggers release of human and mouse neutrophil extracellular traps[J]. Infect Immun, 2012, 80(2):768-777.
doi: 10.1128/IAI.05730-11 pmid: 22104111 |
| [6] |
Simon D, Simon HU, Yousefi S. Extracellular DNA traps in allergic, infectious, and autoimmune diseases[J]. Allergy, 2013, 68(4):409-416.
doi: 10.1111/all.12111 pmid: 23409745 |
| [7] | Goldmann O, Medina E. The expanding world of extracellular traps: not only neutrophils but much more[J]. Front Immunol, 2013, 3:420. |
| [8] |
Abi Abdallah DS, Denkers EY. Neutrophils cast extracellular traps in response to protozoan parasites[J]. Front Immunol, 2012, 3:382.
doi: 10.3389/fimmu.2012.00382 pmid: 23248631 |
| [9] | Wei FR, Yang YT, Wang JY, et al. Leishmania infantum infection stimulates neutrophils to generate extracellular traps in mouse[J]. Chin J Parasitol Parasit Dis, 2019, 37(1):18-22. (in Chinese) |
| (危芙蓉, 杨玥涛, 汪俊云, 等. 婴儿利什曼原虫感染小鼠体内中性粒细胞形成胞外诱捕网能力的研究[J]. 中国寄生虫学与寄生虫病杂志, 2019, 37(1):18-22.) | |
| [10] |
Ventura-Juarez J, Campos-Esparza M, Pacheco-Yepez J, et al. Entamoeba histolytica induces human neutrophils to form NETs[J]. Parasite Immunol, 2016, 38(8):503-509.
doi: 10.1111/pim.12332 pmid: 27138813 |
| [11] | Sousa-Rocha D, Thomaz-Tobias M, Diniz LF, et al. Trypanosoma cruzi and its soluble antigens induce NET release by stimulating Toll-like receptors[J]. PLoS One, 2015, 10(10):e013956. |
| [12] | Zhu H, Lu SQ, Guo ZZ, et al. The establishment of axenic culture of a Sichuan strain of Giardia lamblia[J]. Chin J Parasit Dis Con, 1992, 5(1):42-44. (in Chinese) |
| (祝虹, 卢思奇, 郭增柱, 等. 蓝氏贾第鞭毛虫四川株纯培养的建立[J]. 中国寄生虫病防治杂志, 1992, 5(1):42-44.) | |
| [13] |
Rodríguez-Espinosa O, Rojas-Espinosa O, Moreno-Altamirano MM, et al. Metabolic requirements for neutrophil extracellular traps formation[J]. Immunology, 2015, 145(2):213-224.
doi: 10.1111/imm.12437 pmid: 25545227 |
| [14] |
Park SY, Shrestha S, Youn YJ, et al. Autophagy primes neutrophils for neutrophil extracellular trap formation during Sepsis[J]. Am J Respir Crit Care Med, 2017, 196(5):577-589.
doi: 10.1164/rccm.201603-0596OC |
| [15] |
Armstrong CL, Klaes CK, Vashishta A, et al. Filifactor alocis manipulates human neutrophils affecting their ability to release neutrophil extracellular traps induced by PMA[J]. Innate Immun, 2018, 24(4):210-220.
doi: 10.1177/1753425918767507 |
| [16] |
Zawrotniak M, Rapala-Kozik M. Neutrophil extracellular traps (NETs)-formation and implications[J]. Acta Biochim Pol, 2013, 60(3):277-284.
pmid: 23819131 |
| [17] |
Azzouz L, Cherry A, Riedl M, et al. Relative antibacterial functions of complement and NETs: NETs trap and complement effectively kills bacteria[J]. Mol Immunol, 2018, 97:71-81.
doi: 10.1016/j.molimm.2018.02.019 |
| [18] |
Villagra-Blanco R, Silva LMR, Görtner U, et al. Molecular analyses on Neospora caninum-triggered NETosis in the caprine system[J]. Dev Comp Immunol, 2017, 72:119-127.
doi: S0145-305X(17)30039-3 pmid: 28254622 |
| [19] |
Wei ZK, Wang ZD, Liu X, et al. Toxoplasma gondii triggers neutrophil extracellular traps release in dogs[J]. Front Cell Infect Microbiol, 2020, 10:429.
doi: 10.3389/fcimb.2020.00429 |
| [20] |
de Bont CM, Boelens WC, Pruijn GJM. NETosis, complement, and coagulation: a triangular relationship[J]. Cell Mol Immunol, 2019, 16(1):19-27.
doi: 10.1038/s41423-018-0024-0 |
| [21] |
Guimarães-Costa AB, DeSouza-Vieira TS, Paletta-Silva R, et al. 3′-nucleotidase/nuclease activity allows Leishmania parasites to escape killing by neutrophil extracellular traps[J]. Infect Immun, 2014, 82(4):1732-1740.
doi: 10.1128/IAI.01232-13 pmid: 24516114 |
| [22] |
Freitas-Mesquita AL, Dick CF, Dos-Santos ALA, et al. Cloning, expression and purification of 3′-nucleotidase/nuclease, an enzyme responsible for the Leishmania escape from neutrophil extracellular traps[J]. Mol Biochem Parasitol, 2019, 229:6-14.
doi: 10.1016/j.molbiopara.2019.02.004 |
| [23] |
Mendez J, Sun DL, Tuo WB, et al. Bovine neutrophils form extracellular traps in response to the gastrointestinal parasite Ostertagia ostertagi[J]. Sci Rep, 2018, 8(1):17598.
doi: 10.1038/s41598-018-36070-3 |
| [24] |
Sousa-Rocha D, Thomaz-Tobias M, Diniz LF, et al. Trypanosoma cruzi and its soluble antigens induce NET release by stimulating toll-like receptors[J]. PLoS One, 2015, 10(10):e0139569.
doi: 10.1371/journal.pone.0139569 |
| [25] |
Aley SB, Zimmerman M, Hetsko M, et al. Killing of Giardia lamblia by cryptdins and cationic neutrophil peptides[J]. Infect Immun, 1994, 62(12):5397-5403.
doi: 10.1128/iai.62.12.5397-5403.1994 pmid: 7960119 |
| [26] |
Cotton JA, Bhargava A, Ferraz JG, et al. Giardia duodenalis cathepsin B proteases degrade intestinal epithelial interleukin-8 and attenuate interleukin-8-induced neutrophil chemotaxis[J]. Infect Immun, 2014, 82(7):2772-2787.
doi: 10.1128/IAI.01771-14 pmid: 24733096 |
| [27] |
Araújo NS, Mundim MJ, Gomes MA, et al. Giardia duodenalis: pathological alterations in gerbils, Meriones unguiculatus, infected with different dosages of trophozoites[J]. Exp Parasitol, 2008, 118(4):449-457.
pmid: 18083166 |
| [28] |
Aratani Y. Myeloperoxidase: its role for host defense, inflammation, and neutrophil function[J]. Arch Biochem Biophys, 2018, 640:47-52.
doi: 10.1016/j.abb.2018.01.004 |
| [29] |
Saha P, Yeoh BS, Xiao X, et al. PAD4-dependent NETs generation are indispensable for intestinal clearance of Citrobacter rodentium[J]. Mucosal Immunol, 2019, 12(3):761-771.
doi: 10.1038/s41385-019-0139-3 pmid: 30710097 |
| [30] |
Eghbalzadeh K, Georgi L, Louis T, et al. Compromised anti-inflammatory action of neutrophil extracellular traps in PAD4-deficient mice contributes to aggravated acute inflammation after myocardial infarction[J]. Front Immunol, 2019, 10:2313.
doi: 10.3389/fimmu.2019.02313 pmid: 31632398 |
| [31] |
Thålin C, Daleskog M, Göransson SP, et al. Validation of an enzyme-linked immunosorbent assay for the quantification of citrullinated histone H3 as a marker for neutrophil extracellular traps in human plasma[J]. Immunol Res, 2017, 65(3):706-712.
doi: 10.1007/s12026-017-8905-3 |
| [1] | WANG Dan, HE Zhiquan, LIU Ying, LIU Lingzhi, CHEN Huihui, JIANG Tiantian, JI Penghui, QIAN Dan, YANG Chengyun, ZHANG Hongwei. Molecular identification and genetic tracing of Giardia lamblia isolated from an infected case [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(3): 380-383. |
| [2] | LI Chang, DU Xinyue, YAN Min, WANG Zhaojun. Research advances on the role and mechanism of neutrophil extracellular traps in parasitic infection [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(2): 219-222. |
| [3] | SUN Jiahui, SONG Peng, CHEN Muxin, ZHOU Yan, LIN Lin, CHEN Jiaxu, CAI Yuchun. Expression and functional analysis of recombinant peptidyl-prolyl cis-trans isomerase gene of Babesia microti [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2023, 41(1): 29-35. |
| [4] | YU Ming-chuan, YANG Zhong-wei, WANG Hua-ran, SHI Dan-yang, ZHOU Shu-qing, YIN Jing, SUN Shu-min. Establishment of a Giardia lamblia detection method based on the LAMP microfluidic chip [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2021, 39(3): 402-405. |
| [5] | Hong-yu SONG, Yu-qing LIANG, Rui-qi HUA, Yuan SHI, Ai-guo YANG, Li GUO, Dong-bo YUAN, Yue XIE, Guang-you YANG. Expression, localization and preliminary evaluation of diagnostic value of Echinococcus granulosus phosphoglycerate mutase [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2020, 38(2): 194-201. |
| [6] | Hua LIU, Ning XU, Yu-juan SHEN, Yuan HU, Jian-ping CAO. Infection and genotype of Giardia lamblia among HIV/AIDS patients in Guangxi [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2019, 37(3): 321-325. |
| [7] | Yi-xue YUAN, Guo-xia ZHENG, Dan-dan WANG, Hang ZHUAN, Meng DU, Yun-hua WANG. Cloning and expression of the nucleoside diphosphate kinase gene of Giardia lamblia [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2019, 37(2): 190-193. |
| [8] | Fu-rong WEI, Yue-tao YANG, Jun-yun WANG, Yan-juan WANG, Jia-ming PAN, Jian-ping CAO. Leishmania infantum infection stimulates neutrophils to generate extracellular traps in mouse [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2019, 37(1): 18-22. |
| [9] | SITU Yong-li1,2, SHAO Zheng1, DENG Li1, SUI Xi-xiang1, LI Hai-jian1,3, XU Qin-ying1, . Study on the protease inhibitory activity of TtSerpin1, a serine protease inhibitor from Trichuris trichiura [J]. , 2017, 35(4): 6-342-346. |
| [10] | Le-sheng ZHANG, Lei SUN, Yuan HU, Wen-ci GONG, Yan-juan WANG, yu-juan SHEN, Yu-xin XU, Tian-ping WANG, Jian-ping CAO. Preparation and identification of monoclonal antibodies targeting Giardia lamblia [J]. CHINESE JOURNAL OF PARASITOLOGY AND PARASITIC DISEASES, 2017, 35(2): 160-163. |
| [11] | JIANG Shi-chen, WEI Hai-xia, HE Cheng, DENG Sheng-qun, XIA Jing, PENG Hong-juan*. Secretion and Distribution of Rhoptry Protein 16 during Toxoplasma gondii Invasion into Host Cells [J]. , 2016, 34(3): 2-189-197. |
| [12] | ZHANG Li,LIAO Qi-Bin,HU Lin,CHEN Ai-Yuan,WEI Hai-Xia,PENG Hong-Juan*. Preparation and Purification of Polyclonal Antibody against Toxoplasma gondii Rhoptry Protein 2(ROP2) and its Application in Immunofluorescence Localization [J]. , 2014, 32(1): 7-34-37. |
| [13] | LU Zhi-min1,WANG Yan1,ZHANG Zi-yang2,TANG Hong-wei1,SUO Xun3 *. Evaluation of an Indirect Immunofluorescence Assay Kit for the Dectetion of Anti-Toxoplasma gondii IgG [J]. , 2013, 31(5): 4-346-351. |
| [14] | ZHAOJian-ling*;GAOXing-zheng;QUMing. Killing Effect of Polymorphonuclear Neutrophils on Trichomonas vaginalis [J]. , 2008, 26(5): 10-369. |
| [15] | LIUQing-zhong*;SHENJi-long~**;WANGXue-ong. Immunolocalization of the Signaling Protein l4-3-3 of Schistosoma japonicum [J]. , 2003, 21(6): 4-332. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||